当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Polymer Nanovesicle-Mediated Delivery of MLN8237 Preferentially Inhibits Aurora Kinase A To Target RalA and Anchorage-Independent Growth in Breast Cancer Cells.
Molecular Pharmaceutics ( IF 4.9 ) Pub Date : 2018-06-04 00:00:00 , DOI: 10.1021/acs.molpharmaceut.8b00163 Siddhi Inchanalkar , Nilesh Umakant Deshpande , Vishakha Kasherwal , Manickam Jayakannan , Nagaraj Balasubramanian
Molecular Pharmaceutics ( IF 4.9 ) Pub Date : 2018-06-04 00:00:00 , DOI: 10.1021/acs.molpharmaceut.8b00163 Siddhi Inchanalkar , Nilesh Umakant Deshpande , Vishakha Kasherwal , Manickam Jayakannan , Nagaraj Balasubramanian
|
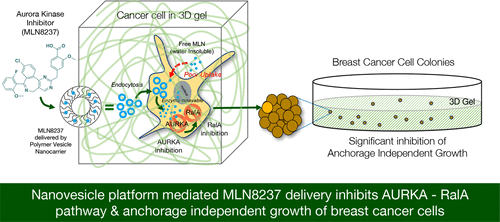
The small GTPase RalA is a known mediator of anchorage-independent growth in cancers and is differentially regulated by adhesion and aurora kinase A (AURKA). Hence, inhibiting AURKA offers a means of specifically targeting RalA (over RalB) in cancer cells. MLN8237 (alisertib) is a known inhibitor of aurora kinases; its specificity for AURKA, however, is compromised by its poor solubility and transport across the cell membrane. A polymer nanovesicle platform is used for the first time to deliver and differentially inhibit AURKA in cancer cells. For this purpose, polysaccharide nanovesicles made from amphiphilic dextran were used as nanocarriers to successfully administer MLN8237 (VMLN) in cancer cells in 2D and 3D microenvironments. These nanovesicles (<200 nm) carry the drug in their intermembrane space with up to 85% of it released by the action of esterase enzyme(s). Lysotracker experiments reveal the polymer nanovesicles localize in the lysosomal compartment of the cell, where they are enzymatically targeted and MLN released in a controlled manner. Rhodamine B fluorophore trapped in the nanovesicles hydrophilic core (VMLN+RhB) allows us to visualize its uptake and localization in cells in a 2D and 3D microenvironment. In breast cancer, MCF-7 cells VMLN inhibits AURKA significantly better than the free drug at low concentrations (0.02–0.04 μM). This ensures that the drug in VMLN at these concentrations can specifically inhibit up to 94% of endogenous AURKA without affecting AURKB. This targeting of AURKA causes the downstream differential inhibition of active RalA (but not RalB). Free MLN8237 at similar concentrations and conditions failed to affect RalA activation. VMLN-mediated inhibition of RalA, in turn, disrupts the anchorage-independent growth of MCF-7 cells supporting a role for the AURKA–RalA crosstalk in mediating the same. These studies not only identify the polysaccharide nanovesicle to be an improved way to efficiently deliver low concentrations of MLN8237 to inhibit AURKA but, in doing so, also help reveal a role for AURKA and its crosstalk with RalA in anchorage-independent growth of MCF-7 cells.
中文翻译:

聚合物纳米囊泡介导的 MLN8237 递送优先抑制极光激酶 A 靶向 RalA 和乳腺癌细胞中的锚定非依赖性生长。
小 GTPase RalA 是癌症中不依赖锚定生长的已知介质,并且受粘附和极光激酶 A (AURKA) 的不同调节。因此,抑制 AURKA 提供了一种在癌细胞中特异性靶向 RalA(超过 RalB)的方法。MLN8237 (alisertib) 是一种已知的极光激酶抑制剂;然而,它对 AURKA 的特异性受到其溶解性差和跨细胞膜转运的影响。聚合物纳米囊泡平台首次用于在癌细胞中传递和差异抑制 AURKA。为此,由两亲性葡聚糖制成的多糖纳米囊泡被用作纳米载体,成功地管理 MLN8237(V MLN) 在 2D 和 3D 微环境中的癌细胞中。这些纳米囊泡(<200 nm)在其膜间空间携带药物,其中高达 85% 的药物通过酯酶的作用释放。Lysotracker 实验揭示了聚合物纳米囊泡位于细胞的溶酶体区室中,在那里它们被酶靶向并以受控方式释放 MLN。被困在纳米囊泡亲水核心 (V MLN+RhB ) 中的罗丹明 B 荧光团使我们能够在 2D 和 3D 微环境中可视化其在细胞中的摄取和定位。在乳腺癌中,MCF-7 细胞 V MLN在低浓度 (0.02–0.04 μM) 下对 AURKA 的抑制效果明显优于游离药物。这确保了 V MLN中的药物在这些浓度下,可特异性抑制高达 94% 的内源性 AURKA,而不影响 AURKB。AURKA 的这种靶向导致活性 RalA(但不是 RalB)的下游差异抑制。类似浓度和条件下的游离 MLN8237 未能影响 RalA 激活。V MLN介导的 RalA 抑制反过来破坏了 MCF-7 细胞的锚定非依赖性生长,支持 AURKA-RalA 串扰在介导相同的作用中的作用。这些研细胞。
更新日期:2018-06-04
中文翻译:

聚合物纳米囊泡介导的 MLN8237 递送优先抑制极光激酶 A 靶向 RalA 和乳腺癌细胞中的锚定非依赖性生长。
小 GTPase RalA 是癌症中不依赖锚定生长的已知介质,并且受粘附和极光激酶 A (AURKA) 的不同调节。因此,抑制 AURKA 提供了一种在癌细胞中特异性靶向 RalA(超过 RalB)的方法。MLN8237 (alisertib) 是一种已知的极光激酶抑制剂;然而,它对 AURKA 的特异性受到其溶解性差和跨细胞膜转运的影响。聚合物纳米囊泡平台首次用于在癌细胞中传递和差异抑制 AURKA。为此,由两亲性葡聚糖制成的多糖纳米囊泡被用作纳米载体,成功地管理 MLN8237(V MLN) 在 2D 和 3D 微环境中的癌细胞中。这些纳米囊泡(<200 nm)在其膜间空间携带药物,其中高达 85% 的药物通过酯酶的作用释放。Lysotracker 实验揭示了聚合物纳米囊泡位于细胞的溶酶体区室中,在那里它们被酶靶向并以受控方式释放 MLN。被困在纳米囊泡亲水核心 (V MLN+RhB ) 中的罗丹明 B 荧光团使我们能够在 2D 和 3D 微环境中可视化其在细胞中的摄取和定位。在乳腺癌中,MCF-7 细胞 V MLN在低浓度 (0.02–0.04 μM) 下对 AURKA 的抑制效果明显优于游离药物。这确保了 V MLN中的药物在这些浓度下,可特异性抑制高达 94% 的内源性 AURKA,而不影响 AURKB。AURKA 的这种靶向导致活性 RalA(但不是 RalB)的下游差异抑制。类似浓度和条件下的游离 MLN8237 未能影响 RalA 激活。V MLN介导的 RalA 抑制反过来破坏了 MCF-7 细胞的锚定非依赖性生长,支持 AURKA-RalA 串扰在介导相同的作用中的作用。这些研细胞。



























 京公网安备 11010802027423号
京公网安备 11010802027423号