当前位置:
X-MOL 学术
›
J. Phys. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Mechanism of Cardiac Tropomyosin Transitions on Filamentous Actin As Revealed by All-Atom Steered Molecular Dynamics Simulations
The Journal of Physical Chemistry Letters ( IF 5.7 ) Pub Date : 2018-06-04 00:00:00 , DOI: 10.1021/acs.jpclett.8b00958 Michael R. Williams 1 , Jil C. Tardiff 2, 3 , Steven D. Schwartz 1
The Journal of Physical Chemistry Letters ( IF 5.7 ) Pub Date : 2018-06-04 00:00:00 , DOI: 10.1021/acs.jpclett.8b00958 Michael R. Williams 1 , Jil C. Tardiff 2, 3 , Steven D. Schwartz 1
Affiliation
|
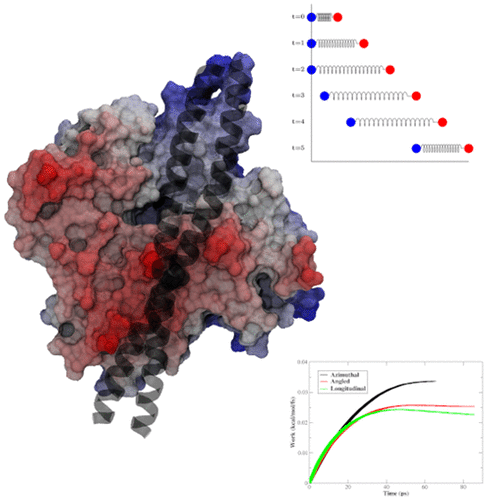
The three-state model of tropomyosin (Tm) positioning along filamentous actin allows for Tm to act as a gate for myosin head binding with actin. The blocked state of Tm prevents myosin binding, while the open state allows for strong binding. Intermediate to this transition is the closed state. The details of the transition from the blocked to the closed state and then finally to the open state by Tm have not been fully accessible to experiment. Utilizing steered molecular dynamics, we investigate the work required to move the Tm strand through the extant set of proposed transitions. We find that an azimuthal motion around the actin filament by Tm is most probable in spite of increased initial energy barrier from the topographical landscape of actin.
中文翻译:

全原子操纵分子动力学模拟揭示的心肌肌球蛋白转变对丝状肌动蛋白的机制。
沿丝状肌动蛋白定位的原肌球蛋白(Tm)的三态模型允许Tm充当肌动蛋白头部与肌动蛋白结合的门。Tm的封闭状态阻止了肌球蛋白的结合,而开放状态则允许牢固的结合。过渡状态的中间状态是封闭状态。从Tm到阻塞状态再到闭合状态再到打开状态的过渡细节尚无法完全用于实验。利用操纵的分子动力学,我们研究了使Tm链穿过现有的拟议过渡集合所需的工作。我们发现,尽管肌动蛋白的地形景观增加了初始能垒,但由Tm围绕肌动蛋白丝进行的方位运动还是最有可能的。
更新日期:2018-06-04
中文翻译:

全原子操纵分子动力学模拟揭示的心肌肌球蛋白转变对丝状肌动蛋白的机制。
沿丝状肌动蛋白定位的原肌球蛋白(Tm)的三态模型允许Tm充当肌动蛋白头部与肌动蛋白结合的门。Tm的封闭状态阻止了肌球蛋白的结合,而开放状态则允许牢固的结合。过渡状态的中间状态是封闭状态。从Tm到阻塞状态再到闭合状态再到打开状态的过渡细节尚无法完全用于实验。利用操纵的分子动力学,我们研究了使Tm链穿过现有的拟议过渡集合所需的工作。我们发现,尽管肌动蛋白的地形景观增加了初始能垒,但由Tm围绕肌动蛋白丝进行的方位运动还是最有可能的。



























 京公网安备 11010802027423号
京公网安备 11010802027423号