当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Chiral Brønsted acid-catalyzed intramolecular SN2′ reaction for enantioselective construction of a quaternary stereogenic center†
Chemical Science ( IF 8.4 ) Pub Date : 2018-06-05 00:00:00 , DOI: 10.1039/c8sc01942h Masahiro Shimizu 1 , Jun Kikuchi 1 , Azusa Kondoh 2 , Masahiro Terada 1
Chemical Science ( IF 8.4 ) Pub Date : 2018-06-05 00:00:00 , DOI: 10.1039/c8sc01942h Masahiro Shimizu 1 , Jun Kikuchi 1 , Azusa Kondoh 2 , Masahiro Terada 1
Affiliation
|
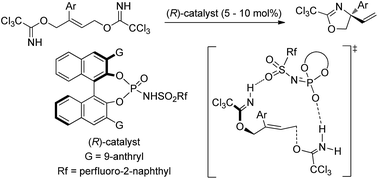
An enantioselective intramolecular anti-SN2′ cyclization reaction for the construction of a quaternary stereogenic center was accomplished through the activation of the leaving group using a binaphthol-derived phosphoramide as the chiral Brønsted acid catalyst. The present allylic substitution reaction is beneficial not only for the regioselective nucleophilic substitution at the multi-substituted site of the double bond but also for controlling the stereochemical outcome because of using a geometrically defined double bond. Indeed, the reaction afforded synthetically useful amino alcohol derivatives having a tetra-substituted carbon center in a highly enantioselective manner in most cases, in which the modification of the sulfonamide unit of the phosphoramide catalyst was demonstrated to improve the enantioselectivity. Experimental and theoretical elucidation of the reaction mechanism suggested that the reaction proceeds through a synchronous anti-SN2′ pathway, although NMR monitoring of the reaction indicated the formation of the phosphorimidate ester via the SN2 reaction of the catalyst with the substrate, which results in catalyst deactivation. Further theoretical studies of the origin of the stereochemical outcome at the generated quaternary stereogenic center were performed. Structural analysis of the transition states at the enantio-determining step revealed that the distinct discrimination of the substituents attached to the geometrically defined double bond is achieved by the anthryl and sulfonamide substituents of the catalyst through the three-point hydrogen bonding interactions and the T-shaped C–H⋯π interactions.
中文翻译:

手性 Brønsted 酸催化的分子内 SN2' 反应用于对映选择性构建四元立体中心†
一种对映选择性分子内抗-S N用于构建四元立体中心的 2' 环化反应是通过使用联萘酚衍生的磷酰胺作为手性布朗斯台德酸催化剂激活离去基团来完成的。本发明的烯丙基取代反应不仅有利于在双键的多取代位点进行区域选择性亲核取代,而且由于使用了几何定义的双键,因此也有利于控制立体化学结果。实际上,该反应在大多数情况下以高度对映选择性的方式提供了具有四取代碳中心的合成有用的氨基醇衍生物,其中磷酰胺催化剂的磺酰胺单元的修饰被证明可以提高对映选择性。抗-S N 2' 途径,尽管反应的 NMR 监测表明通过催化剂与底物的 S N 2 反应形成亚磷酸酯,这导致催化剂失活。对生成的四元立体中心的立体化学结果的起源进行了进一步的理论研究。在对映体确定步骤中过渡态的结构分析表明,连接到几何定义的双键上的取代基的明显区别是通过催化剂的蒽基和磺酰胺取代基通过三点氢键相互作用和 T-形 C-H⋯π 相互作用。
更新日期:2018-06-05
中文翻译:

手性 Brønsted 酸催化的分子内 SN2' 反应用于对映选择性构建四元立体中心†
一种对映选择性分子内抗-S N用于构建四元立体中心的 2' 环化反应是通过使用联萘酚衍生的磷酰胺作为手性布朗斯台德酸催化剂激活离去基团来完成的。本发明的烯丙基取代反应不仅有利于在双键的多取代位点进行区域选择性亲核取代,而且由于使用了几何定义的双键,因此也有利于控制立体化学结果。实际上,该反应在大多数情况下以高度对映选择性的方式提供了具有四取代碳中心的合成有用的氨基醇衍生物,其中磷酰胺催化剂的磺酰胺单元的修饰被证明可以提高对映选择性。抗-S N 2' 途径,尽管反应的 NMR 监测表明通过催化剂与底物的 S N 2 反应形成亚磷酸酯,这导致催化剂失活。对生成的四元立体中心的立体化学结果的起源进行了进一步的理论研究。在对映体确定步骤中过渡态的结构分析表明,连接到几何定义的双键上的取代基的明显区别是通过催化剂的蒽基和磺酰胺取代基通过三点氢键相互作用和 T-形 C-H⋯π 相互作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号