当前位置:
X-MOL 学术
›
Bioconjugate Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis of Azide-Modified Chondroitin Sulfate Precursors: Substrates for "Click"- Conjugation with Fluorescent Labels and Oligonucleotides.
Bioconjugate Chemistry ( IF 4.7 ) Pub Date : 2018-06-15 , DOI: 10.1021/acs.bioconjchem.8b00317 Satish Jadhav 1, 2 , Vijay Gulumkar 1 , Prasannakumar Deshpande 3 , Eleanor T Coffey 3 , Harri Lönnberg 1 , Pasi Virta 1
Bioconjugate Chemistry ( IF 4.7 ) Pub Date : 2018-06-15 , DOI: 10.1021/acs.bioconjchem.8b00317 Satish Jadhav 1, 2 , Vijay Gulumkar 1 , Prasannakumar Deshpande 3 , Eleanor T Coffey 3 , Harri Lönnberg 1 , Pasi Virta 1
Affiliation
|
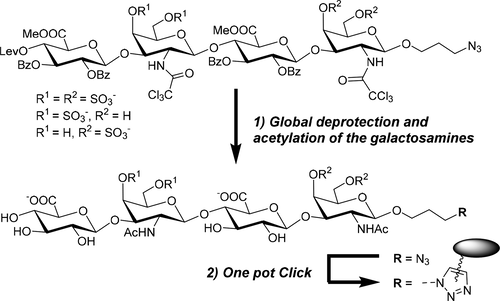
Azidopropyl-modified precursors of chondroitin sulfate (CS) tetrasaccharides have been synthesized, which, after facile conversion to final CS structures, may be conjugated with alkyne-modified target compounds by a one-pot "click"-ligation. RP HPLC was used for the monitoring of the key reaction steps (protecting group manipulation and sulfation) and purification of the CS precursors (as partially protected form, bearing the O-Lev, O-benzoyl, and N-trichloroacetyl groups and methyl esters). Subsequent treatments with aqueous NaOH, concentrated ammonia, and acetic anhydride (i.e., global deprotection and acetylation of the galactosamine units) converted the precursors to final CS structures. The azidopropyl group was exposed to a strain-promoted azide-alkyne cycloaddition (SPAAC) with a dibenzylcyclooctyne-modified carboxyrhodamine dye to give labeled CSs. Conjugation with a 5'-cyclooctyne-modified oligonucleotide was additionally carried out to show the applicability of the precursors for the synthesis of biomolecular hybrids.
中文翻译:

叠氮化物修饰的硫酸软骨素前体的合成:“点击”-与荧光标记和寡核苷酸缀合的底物。
已经合成了软骨素硫酸盐(CS)四糖的叠氮基丙基修饰的前体,可以容易地转化为最终的CS结构,然后通过“一键式”“点击”连接将其与炔烃修饰的目标化合物缀合。RP HPLC用于监测关键反应步骤(保护基操作和硫酸化)和纯化CS前体(作为部分保护形式,带有O-Lev,O-苯甲酰基和N-三氯乙酰基和甲酯) 。随后用NaOH水溶液,浓氨水和乙酸酐处理(即半乳糖胺单元的整体脱保护和乙酰化)将前体转化为最终的CS结构。叠氮基丙基与二苄基环辛炔修饰的羧基若丹明染料接触,经应变促进的叠氮化物-炔烃环加成反应(SPAAC),得到标记的CS。还进行了与5'-环辛炔修饰的寡核苷酸的缀合,以显示前体在生物分子杂合体合成中的适用性。
更新日期:2018-06-01
中文翻译:

叠氮化物修饰的硫酸软骨素前体的合成:“点击”-与荧光标记和寡核苷酸缀合的底物。
已经合成了软骨素硫酸盐(CS)四糖的叠氮基丙基修饰的前体,可以容易地转化为最终的CS结构,然后通过“一键式”“点击”连接将其与炔烃修饰的目标化合物缀合。RP HPLC用于监测关键反应步骤(保护基操作和硫酸化)和纯化CS前体(作为部分保护形式,带有O-Lev,O-苯甲酰基和N-三氯乙酰基和甲酯) 。随后用NaOH水溶液,浓氨水和乙酸酐处理(即半乳糖胺单元的整体脱保护和乙酰化)将前体转化为最终的CS结构。叠氮基丙基与二苄基环辛炔修饰的羧基若丹明染料接触,经应变促进的叠氮化物-炔烃环加成反应(SPAAC),得到标记的CS。还进行了与5'-环辛炔修饰的寡核苷酸的缀合,以显示前体在生物分子杂合体合成中的适用性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号