当前位置:
X-MOL 学术
›
Bioconjugate Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A Quantum Dot-Protein Bioconjugate That Provides for Extracellular Control of Intracellular Drug Release
Bioconjugate Chemistry ( IF 4.7 ) Pub Date : 2018-05-31 00:00:00 , DOI: 10.1021/acs.bioconjchem.8b00357 Lauren D. Field 1 , Scott A. Walper 1 , Kimihiro Susumu 2, 3 , Guillermo Lasarte-Aragones 1, 4 , Eunkeu Oh 2, 3 , Igor L. Medintz 1 , James B. Delehanty 1
Bioconjugate Chemistry ( IF 4.7 ) Pub Date : 2018-05-31 00:00:00 , DOI: 10.1021/acs.bioconjchem.8b00357 Lauren D. Field 1 , Scott A. Walper 1 , Kimihiro Susumu 2, 3 , Guillermo Lasarte-Aragones 1, 4 , Eunkeu Oh 2, 3 , Igor L. Medintz 1 , James B. Delehanty 1
Affiliation
|
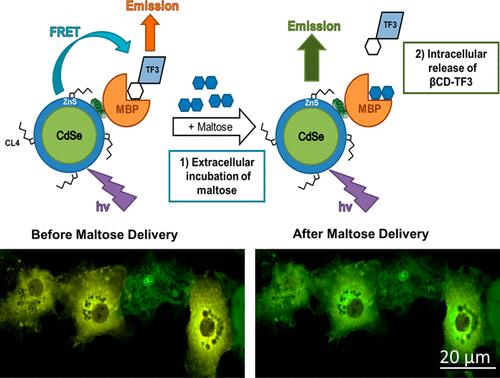
The ability to control the intracellular release of drug cargos from nanobioconjugate delivery scaffolds is critical for the successful implementation of nanoparticle (NP)-mediated drug delivery. This is particularly true for hard NP carriers such as semiconductor quantum dots (QDs) and gold NPs. Here, we report the development of a QD-based multicomponent drug release system that, when delivered to the cytosol of mammalian cells, is triggered to release its drug cargo by the simple addition of a competitive ligand to the extracellular medium. The ensemble construct consists of the central QD scaffold that is decorated with a fixed number of maltose binding proteins (MBPs). The MBP binding site is loaded with dye or drug conjugates of the maltose analogue beta-cyclodextrin (βCD) to yield a QD–MBP−βCD ensemble conjugate. The fidelity of conjugate assembly is monitored by Förster resonance energy transfer (FRET) from the QD donor to the dye/drug acceptor. Microplate-based FRET assays demonstrated that the βCD conjugate was released from the MBP binding pocket by maltose addition with an affinity that matched native MBP–maltose binding interactions. In COS-1 cells, the microinjected assembled conjugates remained stably intact in the cytosol until the addition of maltose to the extracellular medium, which underwent facilitated uptake into the cell. Live cell FRET-based confocal microscopy imaging captured the kinetics of realtime release of the βCD ligand as a function of extracellular maltose concentration. Our results demonstrate the utility of the self-assembled QD–MBP−βCD system to facilitate intracellular drug release that is triggered extracellularly through the simple addition of a well-tolerated nutrient and is not dependent on the use of light, magnetic field, ultrasound, or other traditional methods of stimulated drug release. We expect this extracellularly triggered drug release modality to be useful for the in vitro characterization of new drug candidates intended for systemic delivery/actuation and potentially for on-demand drug release in vivo.
中文翻译:

提供细胞外药物释放的细胞外控制的量子点蛋白质生物共轭物。
控制药物从纳米生物共轭物递送支架向细胞内释放的能力对于成功实施纳米颗粒(NP)介导的药物递送至关重要。对于诸如半导体量子点(QD)和金NP的硬NP载体尤其如此。在这里,我们报告了基于QD的多组分药物释放系统的开发,该系统在递送至哺乳动物细胞的细胞质时,通过将竞争性配体简单添加至细胞外培养基而触发释放其药物。整体构建体由中央QD支架组成,该支架用固定数量的麦芽糖结合蛋白(MBP)装饰。MBP结合位点上装有麦芽糖类似物β-环糊精(βCD)的染料或药物偶联物,以产生QD-MBP-βCD整体偶联物。通过从QD供体到染料/药物受体的Förster共振能量转移(FRET)来监测共轭物组件的保真度。基于微孔板的FRET分析表明,通过添加麦芽糖具有与天然MBP-麦芽糖结合相互作用相匹配的亲和力,βCD缀合物从MBP结合口袋中释放出来。在COS-1细胞中,微注射的组装的结合物在胞质溶胶中保持完整,直到向细胞外培养基中添加麦芽糖,从而促进了细胞摄取。基于活细胞FRET的共聚焦显微镜成像捕获了βCD配体实时释放的动力学,该动力学是细胞外麦芽糖浓度的函数。我们的研究结果表明,自组装QD–MBP-βCD系统可促进细胞内药物的释放,而细胞内药物的释放是通过简单添加良好耐受的营养物而在细胞外触发的,并且不依赖于光,磁场,超声,或其他刺激药物释放的传统方法。我们希望这种细胞外触发的药物释放方式可用于体外候选药物的体外表征,这些候选药物旨在用于全身递送/致动,并可能用于体内按需药物释放。
更新日期:2018-05-31
中文翻译:

提供细胞外药物释放的细胞外控制的量子点蛋白质生物共轭物。
控制药物从纳米生物共轭物递送支架向细胞内释放的能力对于成功实施纳米颗粒(NP)介导的药物递送至关重要。对于诸如半导体量子点(QD)和金NP的硬NP载体尤其如此。在这里,我们报告了基于QD的多组分药物释放系统的开发,该系统在递送至哺乳动物细胞的细胞质时,通过将竞争性配体简单添加至细胞外培养基而触发释放其药物。整体构建体由中央QD支架组成,该支架用固定数量的麦芽糖结合蛋白(MBP)装饰。MBP结合位点上装有麦芽糖类似物β-环糊精(βCD)的染料或药物偶联物,以产生QD-MBP-βCD整体偶联物。通过从QD供体到染料/药物受体的Förster共振能量转移(FRET)来监测共轭物组件的保真度。基于微孔板的FRET分析表明,通过添加麦芽糖具有与天然MBP-麦芽糖结合相互作用相匹配的亲和力,βCD缀合物从MBP结合口袋中释放出来。在COS-1细胞中,微注射的组装的结合物在胞质溶胶中保持完整,直到向细胞外培养基中添加麦芽糖,从而促进了细胞摄取。基于活细胞FRET的共聚焦显微镜成像捕获了βCD配体实时释放的动力学,该动力学是细胞外麦芽糖浓度的函数。我们的研究结果表明,自组装QD–MBP-βCD系统可促进细胞内药物的释放,而细胞内药物的释放是通过简单添加良好耐受的营养物而在细胞外触发的,并且不依赖于光,磁场,超声,或其他刺激药物释放的传统方法。我们希望这种细胞外触发的药物释放方式可用于体外候选药物的体外表征,这些候选药物旨在用于全身递送/致动,并可能用于体内按需药物释放。



























 京公网安备 11010802027423号
京公网安备 11010802027423号