Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Phase-separation mechanism for C-terminal hyperphosphorylation of RNA polymerase II
Nature ( IF 64.8 ) Pub Date : 2018-05-30 , DOI: 10.1038/s41586-018-0174-3 Huasong Lu , Dan Yu , Anders S. Hansen , Sourav Ganguly , Rongdiao Liu , Alec Heckert , Xavier Darzacq , Qiang Zhou
Nature ( IF 64.8 ) Pub Date : 2018-05-30 , DOI: 10.1038/s41586-018-0174-3 Huasong Lu , Dan Yu , Anders S. Hansen , Sourav Ganguly , Rongdiao Liu , Alec Heckert , Xavier Darzacq , Qiang Zhou
|
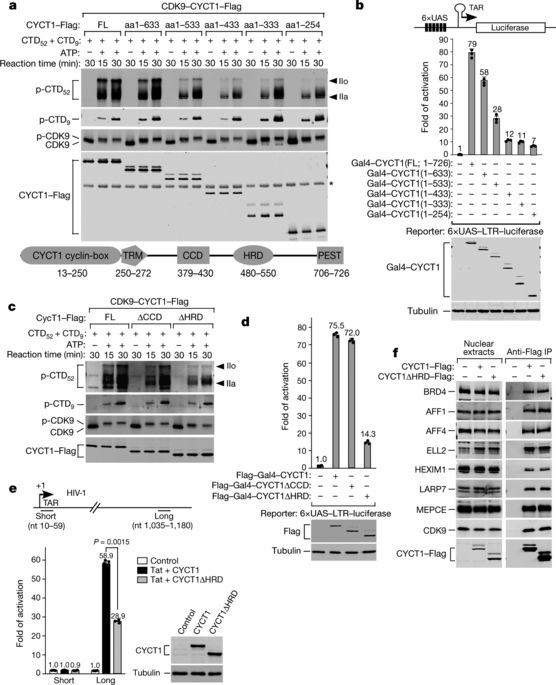
Hyperphosphorylation of the C-terminal domain (CTD) of the RPB1 subunit of human RNA polymerase (Pol) II is essential for transcriptional elongation and mRNA processing1–3. The CTD contains 52 heptapeptide repeats of the consensus sequence YSPTSPS. The highly repetitive nature and abundant possible phosphorylation sites of the CTD exert special constraints on the kinases that catalyse its hyperphosphorylation. Positive transcription elongation factor b (P-TEFb)—which consists of CDK9 and cyclin T1—is known to hyperphosphorylate the CTD and negative elongation factors to stimulate Pol II elongation1,4,5. The sequence determinant on P-TEFb that facilitates this action is currently unknown. Here we identify a histidine-rich domain in cyclin T1 that promotes the hyperphosphorylation of the CTD and stimulation of transcription by CDK9. The histidine-rich domain markedly enhances the binding of P-TEFb to the CTD and functional engagement with target genes in cells. In addition to cyclin T1, at least one other kinase—DYRK1A6—also uses a histidine-rich domain to target and hyperphosphorylate the CTD. As a low-complexity domain, the histidine-rich domain also promotes the formation of phase-separated liquid droplets in vitro, and the localization of P-TEFb to nuclear speckles that display dynamic liquid properties and are sensitive to the disruption of weak hydrophobic interactions. The CTD—which in isolation does not phase separate, despite being a low-complexity domain—is trapped within the cyclin T1 droplets, and this process is enhanced upon pre-phosphorylation by CDK7 of transcription initiation factor TFIIH1–3. By using multivalent interactions to create a phase-separated functional compartment, the histidine-rich domain in kinases targets the CTD into this environment to ensure hyperphosphorylation and efficient elongation of Pol II.The histidine-rich domain of cyclin T1 promotes phase separation into liquid droplets, which facilitates the hyperphosphorylation of the C-terminal domain repeats of RNA polymerase II.
中文翻译:

RNA聚合酶II C末端过度磷酸化的相分离机制
人 RNA 聚合酶 (Pol) II 的 RPB1 亚基的 C 末端结构域 (CTD) 的过度磷酸化对于转录延伸和 mRNA 加工至关重要1-3。CTD 包含共有序列 YSPTSPS 的 52 个七肽重复。CTD 的高度重复性和丰富的可能磷酸化位点对催化其过度磷酸化的激酶施加特殊限制。已知正转录延伸因子 b (P-TEFb)(由 CDK9 和细胞周期蛋白 T1 组成)可使 CTD 过度磷酸化,而负延伸因子可刺激 Pol II 延伸 1,4,5。P-TEFb 上促进这一作用的序列决定因素目前尚不清楚。在这里,我们确定了细胞周期蛋白 T1 中富含组氨酸的结构域,该结构域促进 CTD 的过度磷酸化和 CDK9 的转录刺激。富含组氨酸的结构域显着增强了 P-TEFb 与 CTD 的结合以及与细胞中靶基因的功能结合。除了细胞周期蛋白 T1,至少一种其他激酶——DYRK1A6——也使用富含组氨酸的结构域来靶向 CTD 并使其过度磷酸化。作为一个低复杂度的域,富含组氨酸的域还促进了体外相分离液滴的形成,以及 P-TEFb 定位到显示动态液体特性并对弱疏水相互作用的破坏敏感的核斑点. CTD——尽管是一个低复杂度的结构域,但在隔离时不会相分离——被困在细胞周期蛋白 T1 液滴内,并且在转录起始因子 TFIIH1-3 的 CDK7 预磷酸化后,该过程得到增强。
更新日期:2018-05-30
中文翻译:

RNA聚合酶II C末端过度磷酸化的相分离机制
人 RNA 聚合酶 (Pol) II 的 RPB1 亚基的 C 末端结构域 (CTD) 的过度磷酸化对于转录延伸和 mRNA 加工至关重要1-3。CTD 包含共有序列 YSPTSPS 的 52 个七肽重复。CTD 的高度重复性和丰富的可能磷酸化位点对催化其过度磷酸化的激酶施加特殊限制。已知正转录延伸因子 b (P-TEFb)(由 CDK9 和细胞周期蛋白 T1 组成)可使 CTD 过度磷酸化,而负延伸因子可刺激 Pol II 延伸 1,4,5。P-TEFb 上促进这一作用的序列决定因素目前尚不清楚。在这里,我们确定了细胞周期蛋白 T1 中富含组氨酸的结构域,该结构域促进 CTD 的过度磷酸化和 CDK9 的转录刺激。富含组氨酸的结构域显着增强了 P-TEFb 与 CTD 的结合以及与细胞中靶基因的功能结合。除了细胞周期蛋白 T1,至少一种其他激酶——DYRK1A6——也使用富含组氨酸的结构域来靶向 CTD 并使其过度磷酸化。作为一个低复杂度的域,富含组氨酸的域还促进了体外相分离液滴的形成,以及 P-TEFb 定位到显示动态液体特性并对弱疏水相互作用的破坏敏感的核斑点. CTD——尽管是一个低复杂度的结构域,但在隔离时不会相分离——被困在细胞周期蛋白 T1 液滴内,并且在转录起始因子 TFIIH1-3 的 CDK7 预磷酸化后,该过程得到增强。



























 京公网安备 11010802027423号
京公网安备 11010802027423号