Bioorganic & Medicinal Chemistry Letters ( IF 2.7 ) Pub Date : 2018-05-30 , DOI: 10.1016/j.bmcl.2018.05.057 Ka Yang , Yanling Song , Haibo Xie , Hao Wu , Yi-Ting Wu , Eric D. Leisten , Weiping Tang
|
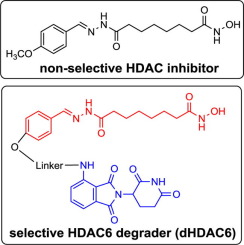
Histone deacetylases (HDACs) decrease the acetylation level of histones and other non-histone proteins. Over expression of HDACs have been observed in cancers and other diseases. Targeted protein degradation by “hijacking” the natural ubiquitin-proteasome-system (UPS) recently emerged as a novel technology to “knock-out” endogenous disease-causing proteins. We applied this strategy to the development of the first small molecule degraders for zinc-dependent HDACs by conjugating non-selective HDAC inhibitors with E3 ubiquitin ligase ligands. Through cell-based assays, we discovered novel bifunctional molecules (dHDAC6) that could selectively degrade HDAC6. Further mechanistic studies indicated that HDAC6 was selectively removed by the UPS.
中文翻译:

第一个小分子组蛋白脱乙酰基酶6(HDAC6)降解物的开发
组蛋白脱乙酰基酶(HDAC)会降低组蛋白和其他非组蛋白的乙酰化水平。在癌症和其他疾病中已经观察到HDAC的过度表达。通过“劫持”天然泛素-蛋白酶体系统(UPS)进行有针对性的蛋白质降解,最近成为一种“剔除”内源性致病蛋白质的新技术。通过将非选择性HDAC抑制剂与E3泛素连接酶配体缀合,我们将该策略应用于了锌依赖性HDAC的第一个小分子降解剂的开发。通过基于细胞的分析,我们发现了可以选择性降解HDAC6的新型双功能分子(dHDAC6)。进一步的机理研究表明,UPS选择性地去除了HDAC6。



























 京公网安备 11010802027423号
京公网安备 11010802027423号