当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Binding of Divalent Metal Ions with Deprotonated Peptides: Do Gas-Phase Anions Parallel the Condensed Phase?
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2018-06-13 , DOI: 10.1021/acs.jpca.8b02926 Robert C Dunbar 1 , Jonathan Martens 2 , Giel Berden 2 , Jos Oomens 2, 3
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2018-06-13 , DOI: 10.1021/acs.jpca.8b02926 Robert C Dunbar 1 , Jonathan Martens 2 , Giel Berden 2 , Jos Oomens 2, 3
Affiliation
|
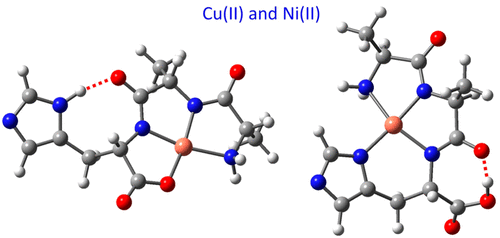
Chelation complexes of the histidine-containing tripeptides HisAlaAla, AlaHisAla, and AlaAlaHis with Ni(II) and Cu(II) having a -1 net charge are characterized in the gas phase by infrared multiple-photon dissociation (IRMPD) spectroscopy and density functional theory calculations. We address the question of whether the gas-phase complexes carry over characteristics from the corresponding condensed-phase species. We focus particularly on three aspects of their structure: (i) square-planar chelation by the deprotonated amide nitrogens around the metal ion (low-spin for the Ni case), (ii) metal-ion coordination of the imidazole side chain nitrogen, and (iii) the exceptional preference for metal-ion chelation by peptides with His in the third position from the N-terminus, as in the amino terminal Cu and Ni (ATCUN) motif. We find that square-planar binding around the metal ion, involving bonds to both deprotonated backbone nitrogens, one of the carboxylate oxygens and the N-terminal nitrogen, is the dominant binding motif for all three isomers. In contrast to the condensed-phase behavior, the dominant mode of binding for all three isomers does not involve the imidazole side chain, which is instead placed outside the coordination zone. Only for the AlaAlaHis isomer, the imidazole-bound structure is also detected as a minority population, as identified from a distinctive short-wavelength IR absorption. The observation that this conformation exists only for AlaAlaHis correlates with condensed-phase behavior at neutral-to-basic pH, in the sense that the isomer with His in the third position is exceptionally disposed to metal ion chelation by four nitrogen atoms (4N) when compared with the other isomers. These results also emphasize the divergence between the conformational stabilities in the gas phase and in solution or crystalline environments: in the gas phase, direct metal binding of the imidazole is overall less favorable than the alternative of a remote imidazole that can act as an intramolecular H-bond donor enhancing the gas-phase stability.
中文翻译:

二价金属离子与去质子化肽的结合:气相阴离子是否与冷凝相平行?
含组氨酸的三肽HisAlaAla,AlaHisAla和AlaAlaHis与具有-1净电荷的Ni(II)和Cu(II)的螯合复合物在气相中通过红外多光子离解(IRMPD)光谱和密度泛函理论进行表征计算。我们解决了气相络合物是否具有相应的冷凝相物种的特征的问题。我们特别关注其结构的三个方面:(i)金属离子周围的去质子化的酰胺氮所形成的方平面螯合(对于Ni情况为低自旋),(ii)咪唑侧链氮的金属离子配位, (iii)与氨基末端的铜和镍(ATCUN)基序一样,His位于N末端第三个位置的肽特别喜欢金属离子螯合。我们发现,围绕金属离子的方平面结合,涉及到去质子化的主链氮,羧酸氧之一和N端氮的键合,是所有三个异构体的主要结合基序。与冷凝相行为相反,所有三个异构体的主要结合方式不涉及咪唑侧链,而该咪唑侧链则位于配位区之外。仅对于AlaAlaHis异构体,与咪唑结合的结构也被检测为少数族群,这是通过独特的短波红外吸收确定的。仅在AlaAlaHis中存在这种构象的观察结果与在中性至碱性pH值下的凝结相行为有关,在某种意义上说,与其他异构体相比,His在第三位的异构体被四个氮原子(4N)特殊地与金属离子螯合。这些结果还强调了气相和溶液或结晶环境中构象稳定性之间的差异:在气相中,咪唑的直接金属键合总体上不如可以充当分子内H的远程咪唑的替代方法那么有利。 -键供体提高了气相的稳定性。
更新日期:2018-05-30
中文翻译:

二价金属离子与去质子化肽的结合:气相阴离子是否与冷凝相平行?
含组氨酸的三肽HisAlaAla,AlaHisAla和AlaAlaHis与具有-1净电荷的Ni(II)和Cu(II)的螯合复合物在气相中通过红外多光子离解(IRMPD)光谱和密度泛函理论进行表征计算。我们解决了气相络合物是否具有相应的冷凝相物种的特征的问题。我们特别关注其结构的三个方面:(i)金属离子周围的去质子化的酰胺氮所形成的方平面螯合(对于Ni情况为低自旋),(ii)咪唑侧链氮的金属离子配位, (iii)与氨基末端的铜和镍(ATCUN)基序一样,His位于N末端第三个位置的肽特别喜欢金属离子螯合。我们发现,围绕金属离子的方平面结合,涉及到去质子化的主链氮,羧酸氧之一和N端氮的键合,是所有三个异构体的主要结合基序。与冷凝相行为相反,所有三个异构体的主要结合方式不涉及咪唑侧链,而该咪唑侧链则位于配位区之外。仅对于AlaAlaHis异构体,与咪唑结合的结构也被检测为少数族群,这是通过独特的短波红外吸收确定的。仅在AlaAlaHis中存在这种构象的观察结果与在中性至碱性pH值下的凝结相行为有关,在某种意义上说,与其他异构体相比,His在第三位的异构体被四个氮原子(4N)特殊地与金属离子螯合。这些结果还强调了气相和溶液或结晶环境中构象稳定性之间的差异:在气相中,咪唑的直接金属键合总体上不如可以充当分子内H的远程咪唑的替代方法那么有利。 -键供体提高了气相的稳定性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号