Molecular Catalysis ( IF 4.6 ) Pub Date : 2018-05-29 , DOI: 10.1016/j.mcat.2018.05.023 Akihiro Endo , Takaaki Kurinomaru , Kentaro Shiraki
|
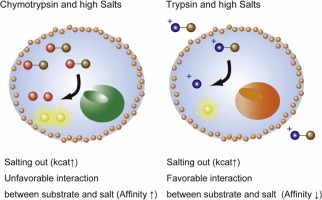
α-Chymotrypsin (CHT) is a serine protease that hydrolyzes peptide bonds at the carboxyl end of hydrophobic amino acids. It has been shown that the enzyme activity of CHT increases one order of magnitude in the presence of some types of amine compounds and inorganic ions. Here we show that hyperactivation occurs for several kinds of serine proteases in the presence of a kosmotrope. Among the eight model enzymes, the catalytic activities of four serine protease increased 1.3–30-fold by the addition of 1.5 M sodium sulfate. Enzyme kinetics and circular dichroism analyses revealed that the hyperactivation is caused by both increased kcat and decreased KM, which reflect stabilization of the active form of the enzyme and the affinity between the enzyme and hydrophobic substrate, respectively. These findings demonstrate the effectiveness of this simple method for enzyme activation without cost and contribute to our fundamental understanding of enzyme activity in solution.
中文翻译:

Hofmeister效应使丝氨酸蛋白酶过度活化
α-胰凝乳蛋白酶(CHT)是一种丝氨酸蛋白酶,可水解疏水氨基酸羧基端的肽键。已经表明,在某些类型的胺化合物和无机离子的存在下,CHT的酶活性增加了一个数量级。在这里,我们显示在大同溶菌的存在下,几种丝氨酸蛋白酶都发生了过度活化。在这八种模型酶中,四种丝氨酸蛋白酶的催化活性通过添加1.5 M硫酸钠而增加了1.3–30倍。酶动力学和圆二色性分析表明,过度激活是由增加的k cat和减少的K M引起的分别反映了酶活性形式的稳定性以及酶与疏水性底物之间的亲和力。这些发现证明了这种简单的酶活化方法的有效性,而无需花费任何费用,并且有助于我们对溶液中酶的活性有基本的了解。


























 京公网安备 11010802027423号
京公网安备 11010802027423号