当前位置:
X-MOL 学术
›
J. Phys. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The Clathrate–Water Interface Is Oleophilic
The Journal of Physical Chemistry Letters ( IF 5.7 ) Pub Date : 2018-05-29 00:00:00 , DOI: 10.1021/acs.jpclett.8b01210 Andressa A. Bertolazzo 1, 2 , Pavithra M. Naullage 1 , Baron Peters 3 , Valeria Molinero 1
The Journal of Physical Chemistry Letters ( IF 5.7 ) Pub Date : 2018-05-29 00:00:00 , DOI: 10.1021/acs.jpclett.8b01210 Andressa A. Bertolazzo 1, 2 , Pavithra M. Naullage 1 , Baron Peters 3 , Valeria Molinero 1
Affiliation
|
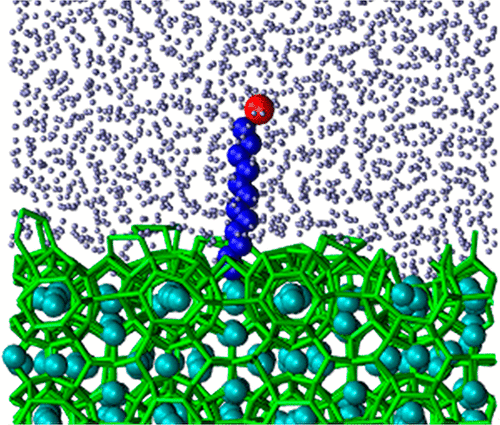
The slow nucleation of clathrate hydrates is a central challenge for their use in the storage and transportation of natural gas. Molecules that strongly adsorb to the clathrate–water interface decrease the crystal–water surface tension, lowering the barrier for clathrate nucleation. Surfactants are widely used to promote the nucleation and growth of clathrate hydrates. It has been proposed that these amphiphilic molecules bind to the clathrate surface via hydrogen bonding. However, recent studies reveal that PVCap, an amphiphilic polymer, binds to clathrates through hydrophobic moieties. Here we use molecular dynamic simulations and theory to investigate the mode and strength of binding of surfactants to the clathrate–water interface and their effect on the nucleation rate. We find that the surfactants bind to the clathrate–water interface exclusively through their hydrophobic tails. The binding is strong, driven by the entropy of dehydration of the alkyl chain, as it penetrates empty cavities at the hydrate surface. The hydrophobic attraction of alkyl groups to the clathrate surface also results in strong adsorption of alkanes. We identify two regimes for the binding of surfactants as a function of their density at the hydrate surface, which we interpret to correspond to the two steps of the Langmuir adsorption isotherm observed in experiments. Our results indicate that hydrophobic attraction to the clathrate–water interface is key for the design of soluble additives that promote the nucleation of hydrates. We use the calculated adsorption coefficients to estimate the concentration of sodium dodecyl sulfate (SDS) required to reach nucleation rates for methane hydrate consistent with those measured in experiments. To our knowledge, this study is the first to quantify the effect of surfactant concentration in the nucleation rate of clathrate hydrates.
中文翻译:

包合物-水的界面是亲油的
笼形水合物的缓慢成核是将其用于天然气储存和运输的主要挑战。强烈吸附到笼形物-水界面的分子会降低晶体-水的表面张力,从而降低笼形物成核的壁垒。表面活性剂被广泛用于促进笼形水合物的成核和生长。已经提出这些两亲分子通过氢键结合到包合物表面。但是,最近的研究表明,两亲聚合物PVCap通过疏水部分与包合物结合。在这里,我们使用分子动力学模拟和理论研究表面活性剂与包合物-水界面结合的方式和强度,以及它们对成核速率的影响。我们发现表面活性剂仅通过其疏水性尾部与包合物-水界面结合。由于烷基链的渗透熵穿透了水合物表面的空洞,因此结合力很强,这是由烷基链的脱水熵驱动的。烷基对包合物表面的疏水吸引也导致烷烃的强烈吸附。我们确定了两种表面活性剂结合方式,它们是它们在水合物表面密度的函数,我们解释为与实验中观察到的Langmuir吸附等温线的两个步骤相对应。我们的结果表明,对包合物-水界面的疏水吸引力是促进水合物成核的可溶性添加剂设计的关键。我们使用计算出的吸附系数来估算达到甲烷水合物成核速率所需的十二烷基硫酸钠(SDS)浓度,该浓度与实验中测得的一致。据我们所知,这项研究是第一个量化表面活性剂浓度对包合物水合物成核速率的影响的研究。
更新日期:2018-05-29
中文翻译:

包合物-水的界面是亲油的
笼形水合物的缓慢成核是将其用于天然气储存和运输的主要挑战。强烈吸附到笼形物-水界面的分子会降低晶体-水的表面张力,从而降低笼形物成核的壁垒。表面活性剂被广泛用于促进笼形水合物的成核和生长。已经提出这些两亲分子通过氢键结合到包合物表面。但是,最近的研究表明,两亲聚合物PVCap通过疏水部分与包合物结合。在这里,我们使用分子动力学模拟和理论研究表面活性剂与包合物-水界面结合的方式和强度,以及它们对成核速率的影响。我们发现表面活性剂仅通过其疏水性尾部与包合物-水界面结合。由于烷基链的渗透熵穿透了水合物表面的空洞,因此结合力很强,这是由烷基链的脱水熵驱动的。烷基对包合物表面的疏水吸引也导致烷烃的强烈吸附。我们确定了两种表面活性剂结合方式,它们是它们在水合物表面密度的函数,我们解释为与实验中观察到的Langmuir吸附等温线的两个步骤相对应。我们的结果表明,对包合物-水界面的疏水吸引力是促进水合物成核的可溶性添加剂设计的关键。我们使用计算出的吸附系数来估算达到甲烷水合物成核速率所需的十二烷基硫酸钠(SDS)浓度,该浓度与实验中测得的一致。据我们所知,这项研究是第一个量化表面活性剂浓度对包合物水合物成核速率的影响的研究。



























 京公网安备 11010802027423号
京公网安备 11010802027423号