当前位置:
X-MOL 学术
›
J. Phys. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Filling Ices with Helium and the Formation of Helium Clathrate Hydrate
The Journal of Physical Chemistry Letters ( IF 5.7 ) Pub Date : 2018-05-29 00:00:00 , DOI: 10.1021/acs.jpclett.8b01423 Werner F. Kuhs 1 , Thomas C. Hansen 2 , Andrzej Falenty 1
The Journal of Physical Chemistry Letters ( IF 5.7 ) Pub Date : 2018-05-29 00:00:00 , DOI: 10.1021/acs.jpclett.8b01423 Werner F. Kuhs 1 , Thomas C. Hansen 2 , Andrzej Falenty 1
Affiliation
|
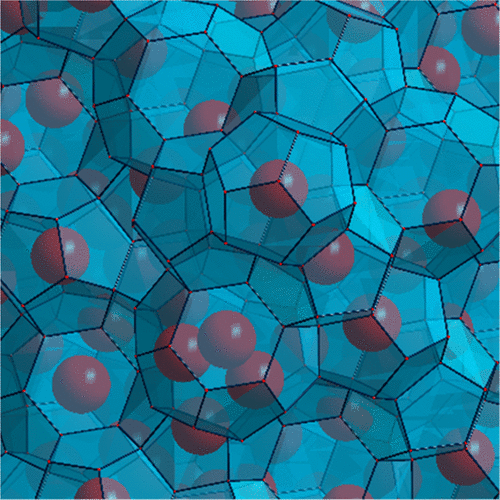
We have formed the long-sought He-clathrate. This was achieved by refilling helium into ice XVI, opening a new synthesis route for exotic forms of clathrate hydrates. The process was followed by neutron diffraction; structures and cage fillings were established. The stabilizing attractive van der Waals interactions are enhanced by multiple cage fillings with theoretically up to four helium atoms per large cage and up to one per small cage; He-clathrate hydrates can be considered as a solid-state equivalent of the clustering of small apolar entities dissolved in the liquid state of water. Unlike most other guests, helium easily enters and leaves the water cages at temperatures well below 100 K, hampering applications as a gas storage material. Despite the weak dispersive interactions, the inclusion of helium has a very significant effect on lattice constants; this is also established for helium inclusion in ice Ih and suggests that lattice parameters are arguably the most sensitive measure to gauge dispersive water–gas interactions.
中文翻译:

用氦填充冰和氦包合物水合物的形成
我们已经形成了人们期盼已久的He-clathrate。这是通过将氦气重新填充到冰XVI中,为异形形式的笼形水合物开辟一条新的合成路线来实现的。此过程之后进行中子衍射;然后进行中子衍射。建立了结构和笼子填充物。稳定的有吸引力的范德华相互作用通过多个笼填充物增强,理论上每个大笼子最多容纳四个氦原子,每个小笼子最多容纳一个氦原子;He-clathate水合物可以看作是溶解在液态水中的非极性小实体簇的固态等效物。与大多数其他客人不同,氦气在温度远低于100 K的情况下很容易进入和离开水笼,阻碍了其作为储气材料的应用。尽管色散相互作用较弱,氦的加入对晶格常数有非常重要的影响;对于冰中的氦气夹杂物也建立了这一点,这表明晶格参数可以说是衡量分散的水-气相互作用的最灵敏的指标。
更新日期:2018-05-29
中文翻译:

用氦填充冰和氦包合物水合物的形成
我们已经形成了人们期盼已久的He-clathrate。这是通过将氦气重新填充到冰XVI中,为异形形式的笼形水合物开辟一条新的合成路线来实现的。此过程之后进行中子衍射;然后进行中子衍射。建立了结构和笼子填充物。稳定的有吸引力的范德华相互作用通过多个笼填充物增强,理论上每个大笼子最多容纳四个氦原子,每个小笼子最多容纳一个氦原子;He-clathate水合物可以看作是溶解在液态水中的非极性小实体簇的固态等效物。与大多数其他客人不同,氦气在温度远低于100 K的情况下很容易进入和离开水笼,阻碍了其作为储气材料的应用。尽管色散相互作用较弱,氦的加入对晶格常数有非常重要的影响;对于冰中的氦气夹杂物也建立了这一点,这表明晶格参数可以说是衡量分散的水-气相互作用的最灵敏的指标。


























 京公网安备 11010802027423号
京公网安备 11010802027423号