当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Effect of Noncanonical Amino Acids on Protein-Carbohydrate Interactions: Structure, Dynamics, and Carbohydrate Affinity of a Lectin Engineered with Fluorinated Tryptophan Analogs.
ACS Chemical Biology ( IF 4 ) Pub Date : 2018-06-12 , DOI: 10.1021/acschembio.8b00377 Felix Tobola 1, 2 , Mickael Lelimousin 3 , Annabelle Varrot 3 , Emilie Gillon 3 , Barbara Darnhofer 1, 4, 5 , Ola Blixt 6 , Ruth Birner-Gruenberger 1, 4, 5 , Anne Imberty 3 , Birgit Wiltschi 1
ACS Chemical Biology ( IF 4 ) Pub Date : 2018-06-12 , DOI: 10.1021/acschembio.8b00377 Felix Tobola 1, 2 , Mickael Lelimousin 3 , Annabelle Varrot 3 , Emilie Gillon 3 , Barbara Darnhofer 1, 4, 5 , Ola Blixt 6 , Ruth Birner-Gruenberger 1, 4, 5 , Anne Imberty 3 , Birgit Wiltschi 1
Affiliation
|
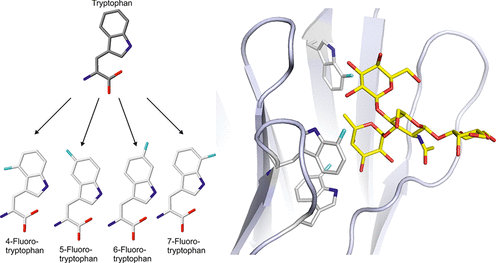
Protein-carbohydrate interactions play crucial roles in biology. Understanding and modifying these interactions is of major interest for fighting many diseases. We took a synthetic biology approach and incorporated noncanonical amino acids into a bacterial lectin to modulate its interactions with carbohydrates. We focused on tryptophan, which is prevalent in carbohydrate binding sites. The exchange of the tryptophan residues with analogs fluorinated at different positions resulted in three distinctly fluorinated variants of the lectin from Ralstonia solanacearum. We observed differences in stability and affinity toward fucosylated glycans and rationalized them by X-ray and modeling studies. While fluorination decreased the aromaticity of the indole ring and, therefore, the strength of carbohydrate-aromatic interactions, additional weak hydrogen bonds were formed between fluorine and the ligand hydroxyl groups. Our approach opens new possibilities to engineer carbohydrate receptors.
中文翻译:

非规范氨基酸对蛋白质-碳水化合物相互作用的影响:结构,动力学和碳水化合物亲和力的氟化色氨酸类似物工程化的亲和力。
蛋白质与碳水化合物的相互作用在生物学中起着至关重要的作用。了解和修改这些相互作用对于抵抗多种疾病具有重大意义。我们采用了合成生物学方法,并将非规范氨基酸掺入细菌凝集素中,以调节其与碳水化合物的相互作用。我们专注于色氨酸,色氨酸普遍存在于碳水化合物结合位点。色氨酸残基与在不同位置处氟化的类似物的交换导致来自青枯菌(Ralstonia solanacearum)的凝集素的三个明显氟化的变体。我们观察了对岩藻糖基化聚糖的稳定性和亲和力的差异,并通过X射线和模型研究对其进行了合理化。氟化会降低吲哚环的芳香性,从而降低碳水化合物与芳香族化合物相互作用的强度,在氟和配体羟基之间形成了另外的弱氢键。我们的方法为工程化碳水化合物受体开辟了新的可能性。
更新日期:2018-05-29
中文翻译:

非规范氨基酸对蛋白质-碳水化合物相互作用的影响:结构,动力学和碳水化合物亲和力的氟化色氨酸类似物工程化的亲和力。
蛋白质与碳水化合物的相互作用在生物学中起着至关重要的作用。了解和修改这些相互作用对于抵抗多种疾病具有重大意义。我们采用了合成生物学方法,并将非规范氨基酸掺入细菌凝集素中,以调节其与碳水化合物的相互作用。我们专注于色氨酸,色氨酸普遍存在于碳水化合物结合位点。色氨酸残基与在不同位置处氟化的类似物的交换导致来自青枯菌(Ralstonia solanacearum)的凝集素的三个明显氟化的变体。我们观察了对岩藻糖基化聚糖的稳定性和亲和力的差异,并通过X射线和模型研究对其进行了合理化。氟化会降低吲哚环的芳香性,从而降低碳水化合物与芳香族化合物相互作用的强度,在氟和配体羟基之间形成了另外的弱氢键。我们的方法为工程化碳水化合物受体开辟了新的可能性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号