Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Selective Preparation of C 2v -Symmetric Hexaphenylbenzene Derivatives through Sequential Suzuki Coupling
Synlett ( IF 2 ) Pub Date : 2018-05-29 , DOI: 10.1055/s-0037-1610024 Tatsuo Kojima 1 , Shuichi Hiraoka 1 , Kazuho Ogata 1
Synlett ( IF 2 ) Pub Date : 2018-05-29 , DOI: 10.1055/s-0037-1610024 Tatsuo Kojima 1 , Shuichi Hiraoka 1 , Kazuho Ogata 1
Affiliation
|
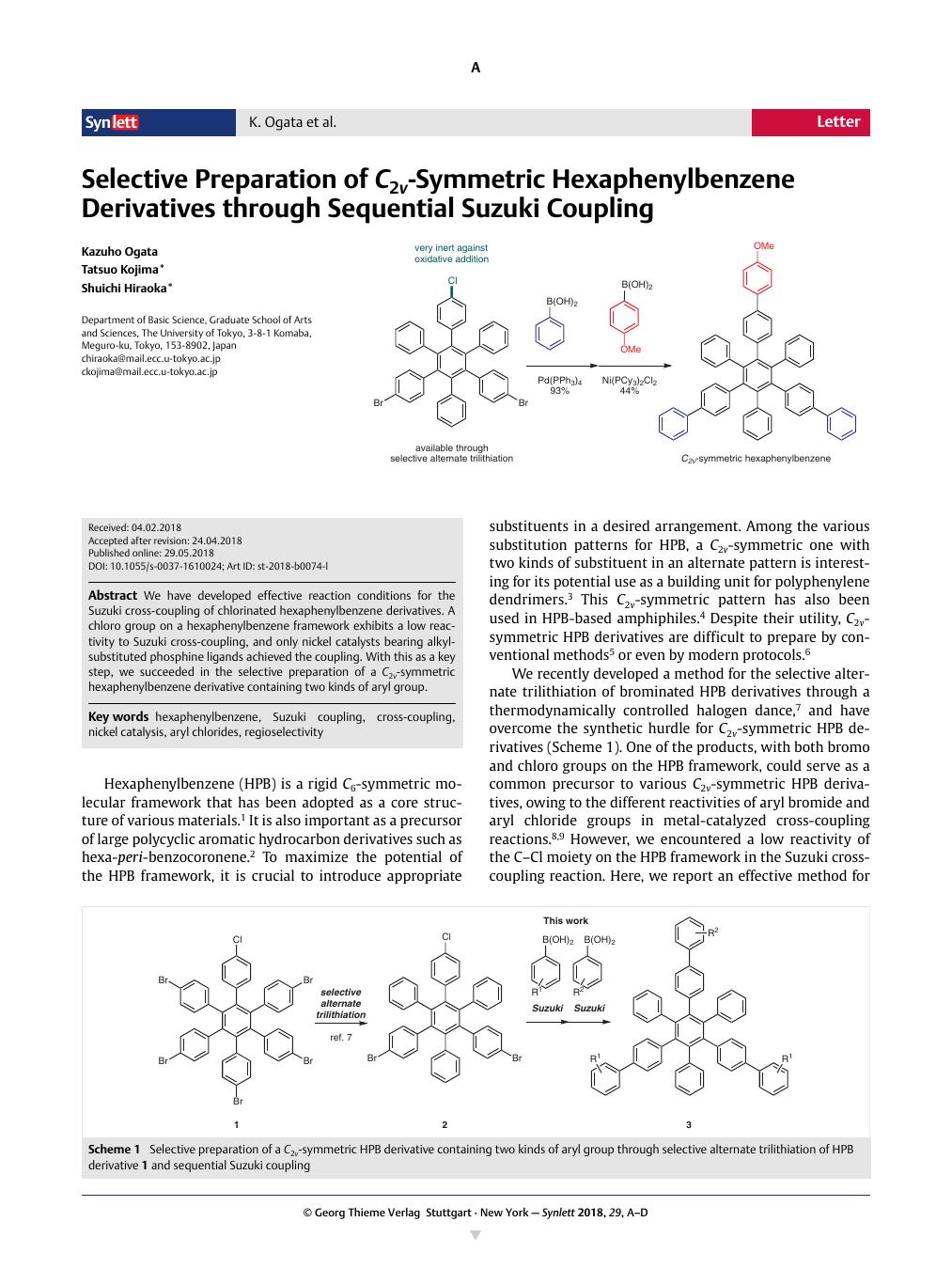
We have developed effective reaction conditions for the Suzuki cross-coupling of chlorinated hexaphenylbenzene derivatives. A chloro group on a hexaphenylbenzene framework exhibits a low reactivity to Suzuki cross-coupling, and only nickel catalysts bearing alkyl-substituted phosphine ligands achieved the coupling. With this as a key step, we succeeded in the selective preparation of a C 2 v -symmetric hexaphenylbenzene derivative containing two kinds of aryl group.
中文翻译:

通过顺序 Suzuki 偶联选择性制备 C 2v -对称六苯基苯衍生物
我们已经为氯化六苯基苯衍生物的 Suzuki 交叉偶联开发了有效的反应条件。六苯基苯骨架上的氯基团对 Suzuki 交叉偶联的反应性较低,只有带有烷基取代膦配体的镍催化剂才能实现偶联。以此为关键步骤,我们成功地选择性制备了含有两种芳基的C 2 v 对称六苯基苯衍生物。
更新日期:2018-05-29
中文翻译:

通过顺序 Suzuki 偶联选择性制备 C 2v -对称六苯基苯衍生物
我们已经为氯化六苯基苯衍生物的 Suzuki 交叉偶联开发了有效的反应条件。六苯基苯骨架上的氯基团对 Suzuki 交叉偶联的反应性较低,只有带有烷基取代膦配体的镍催化剂才能实现偶联。以此为关键步骤,我们成功地选择性制备了含有两种芳基的C 2 v 对称六苯基苯衍生物。



























 京公网安备 11010802027423号
京公网安备 11010802027423号