Journal of Catalysis ( IF 7.3 ) Pub Date : 2018-05-26 , DOI: 10.1016/j.jcat.2018.04.029 Yuki Tsuji , Kiya Ogasawara , Masaaki Kitano , Kazuhisa Kishida , Hitoshi Abe , Yasuhiro Niwa , Toshiharu Yokoyama , Michikazu Hara , Hideo Hosono
|
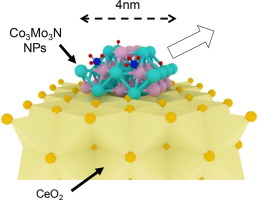
Co-Mo bimetallic nanoparticles (NPs) with various compositions were prepared on a CeO2 support (Co-Mo/CeO2) by sodium naphthalenide driven reduction. The Co-Mo/CeO2 catalyst exhibits much higher activity for ammonia synthesis than monometallic catalysts such as Co/CeO2 and Mo/CeO2, and the optimum activity is obtained at Co:Mo = 4:6. X-ray absorption fine structure (XAFS) analyses reveal that Co3Mo3N NPs are formed after ammonia synthesis reaction. Nitrogen temperature programmed desorption (N2-TPD) measurements indicate that the Co-Mo/CeO2 catalyst possesses a large number of nitrogen adsorption sites with an intermediate nitrogen adsorption energy between that of Co and Mo. Furthermore, nitrogen vacancy sites, which are active sites for N2 dissociation, are more easily formed on the Co-Mo/CeO2 catalyst than on bulk-type Co3Mo3N. As a consequence, the turnover frequency of the Co-Mo/CeO2 catalyst is much higher than that of bulk-type Co3Mo3N and is comparable to that of the efficient Ru/CeO2 catalyst.
中文翻译:

通过萘钠还原制备的Co-Mo双金属纳米颗粒催化剂控制氮的活化能力
通过萘钠驱动的还原,在CeO 2载体(Co-Mo / CeO 2)上制备了具有各种组成的Co-Mo双金属纳米颗粒(NPs)。与单金属催化剂如Co / CeO 2和Mo / CeO 2相比,Co-Mo / CeO 2催化剂显示出更高的氨合成活性,并且在Co:Mo = 4:6时获得了最佳活性。X射线吸收精细结构(XAFS)分析表明,氨合成反应后形成了Co 3 Mo 3 N NPs。氮气程序升温脱附(N 2 -TPD)测量表明Co-Mo / CeO 2催化剂具有大量的氮吸附位,其氮吸附能介于Co和Mo之间。此外,作为N 2离解的活性位的氮空位更容易在Co-Mo / CeO 2催化剂上形成。比批量型Co 3沫3 N.因此,在Co-Mo系/铈的周转频率2催化剂比的批量型Co高得多的3沫3 N和比得上有效的Ru / CeO 2催化剂。



























 京公网安备 11010802027423号
京公网安备 11010802027423号