Journal of Catalysis ( IF 7.3 ) Pub Date : 2018-05-26 , DOI: 10.1016/j.jcat.2018.04.026 Leon G.A. van de Water , Sam K. Wilkinson , Richard A.P. Smith , Michael J. Watson
|
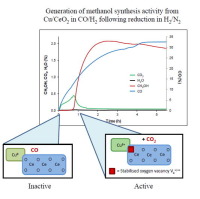
Understanding copper-based methanol synthesis catalysts as a function of catalyst type and applied reaction conditions is an area of active industrial and academic research. In this work, results of a methanol synthesis study over a Cu/CeO2 catalyst using CO/H2 feeds are presented, using catalyst performance data combined with catalyst characterisation information (DRIFTS, XPS, TPR, XRD) obtained during start-up and under steady state methanol synthesis conditions. The results indicate that the active site and reaction mechanism for methanol synthesis over Cu/CeO2 are different from the conventional Cu/ZnO/Al2O3 catalyst, with CO, rather than CO2, being the carbon source for methanol. Fixed-bed micro reactors were employed to obtain catalyst performance data in discrete bed sectors using experimental spatial discretisation methods. Methanol synthesis activity over Cu/CeO2 from CO/H2 is preceded by transient CO2 formation, with the onset of methanol synthesis activity observed when the CO2 formation reaches its peak. Considerable differences between reactor bed sectors are observed during start-up, with CO2 formation, CO2 re-adsorption (as surface carbonates and formates), methanol formation and transient methanol decomposition occurring to different degrees and at different time scales.
The interfaces of defective CeO2−x in contact with highly dispersed copper particles form the active site of the catalyst under steady-state methanol synthesis conditions, with the copper oxidation state being a combination of 0 and +1 states. The CeO2−x defect sites are formed during start-up upon reaction of CeO2 with CO. The CO2 thus formed leads to catalyst deactivation due to build-up of carbonate and formate species on the catalyst surface. A gradient of progressive poisoning/deactivation is observed going further down the catalyst bed, where the level of deactivation can be controlled by modifying the CO content in the feed. CO2 thus acts as a poison for the Cu/CeO2 catalyst, and addition of even low levels of CO2 to the feed leads to an evenly deactivated catalyst. During start-up in CO/H2, transient CO2 and H2O formation is also observed in the case of Cu/ZnO/Al2O3, ascribed to partial reduction of ZnO, similar to the partial reduction of CeO2 as seen in Cu/CeO2. The key difference between the two systems is the absence of CO2 and H2O re-adsorption on Cu/ZnO/Al2O3, resulting in stable methanol production distributed equally over the catalyst bed. Introduction of CO2 to the CO/H2 feed in case of Cu/ZnO/Al2O3 leads to reduction of Cu+ to Cu0 and a change in methanol synthesis mechanism from CO to CO2 hydrogenation.
中文翻译:

了解在Cu / CeO 2催化剂上由CO / H 2进料合成甲醇
了解铜基甲醇合成催化剂与催化剂类型和所应用反应条件的关系是活跃的工业和学术研究领域。在这项工作中,通过使用催化剂性能数据以及在启动和启动过程中获得的催化剂表征信息(DRIFTS,XPS,TPR,XRD),结合使用CO / H 2进料的Cu / CeO 2催化剂,进行了甲醇合成研究的结果。在稳态甲醇合成条件下。结果表明,Cu / CeO 2上甲醇合成的活性部位和反应机理与常规的Cu / ZnO / Al 2 O 3催化剂不同,只是采用CO而不是CO 2。,是甲醇的碳源。使用实验性空间离散化方法,使用固定床微型反应器来获得离散床扇区中的催化剂性能数据。来自CO / H 2的Cu / CeO 2的甲醇合成活性先于短暂的CO 2形成,当CO 2的形成达到峰值时观察到甲醇合成活性的开始。在启动过程中观察到反应器床层区段之间的显着差异,其中CO 2的形成,CO 2的再吸附(作为表面碳酸盐和甲酸盐),甲醇的形成和瞬时甲醇的分解在不同的程度和时间范围内发生。
有缺陷的CeO 2-x与高度分散的铜颗粒接触的界面在稳态甲醇合成条件下形成了催化剂的活性位点,铜的氧化态是0和+1状态的组合。CeO 2-x缺陷部位是在CeO 2与CO反应启动时形成的。由于碳酸盐和甲酸盐类物质在催化剂表面的积聚,因此形成的CO 2导致催化剂失活。在催化剂床的下方观察到逐渐中毒/失活的梯度,在失活水平中可以通过改变进料中的CO含量来控制失活水平。因此,CO 2充当Cu / CeO 2的毒药催化剂,甚至向进料中加入低含量的CO 2都会导致催化剂均匀失活。在启动期间在CO / H 2,瞬态CO 2和H 2 ö形成在Cu的情况下,也观察到/氧化锌/铝2 ö 3,归因于部分还原的ZnO,类似的CeO的部分还原2作为见于Cu / CeO 2。这两个系统之间的主要区别在于在Cu / ZnO / Al 2 O 3上不存在CO 2和H 2 O的再吸附,从而使稳定的甲醇生产均匀地分布在催化剂床上。引入CO 2在Cu / ZnO / Al 2 O 3的情况下,向CO / H 2进料中引入CO / H 2导致Cu +还原为Cu 0以及甲醇合成机理从CO加氢变为CO 2的改变。



























 京公网安备 11010802027423号
京公网安备 11010802027423号