Journal of Inorganic Biochemistry ( IF 3.9 ) Pub Date : 2018-05-26 , DOI: 10.1016/j.jinorgbio.2018.05.016 André M.N. Silva , João T.S. Coimbra , Maria M. Castro , Ângela Oliveira , Natércia F. Brás , Pedro A. Fernandes , Maria J. Ramos , Maria Rangel
|
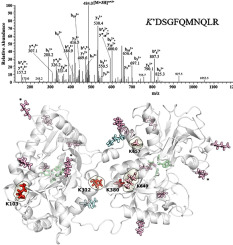
Understanding the effect of glycation on the function of transferrin, the systemic iron transporter, is fundamental to fully grasp the mechanisms leading to the loss of iron homeostasis observed in diabetes mellitus (DM). The spontaneous reaction with protein amino groups is one of the main causes of glucose toxicity, but the site specificity of this reaction is still poorly understood. Here in, an in vitro approach was used to study human holo-transferrin glycation in detail. Lysine residues 103, 312 and 380 proved to be the most reactive sites, and overall glycation specificity was found to be remarkably different from that described for apo-transferrin. A computational biochemistry approach was subsequently applied to rationalize lysine reactivity. Even though pKa values, solvent accessible surface area, hydrogen bonds or the presence of nearby charged/polar residues could be related to lysine reactivity, these parameters do not suffice to describe glycation site specificity in holo-transferrin. Furthermore, analysis of the most reactive residues suggests that the correct lysine side chain orientation may play a fundamental role in reactivity. Nevertheless, in holo-transferrin, glycation occurs away from the iron-binding sites and, despite the observed iron release, the modification of apo-transferrin should play a more relevant role for the loss of iron-binding capacity observed in the blood serum of DM patients.
中文翻译:

测定人全转铁蛋白的糖基化位点特异性
了解糖基化对全身性铁转运蛋白转铁蛋白功能的影响,是全面掌握导致糖尿病(DM)中铁稳态失衡的机制的基础。与蛋白质氨基的自发反应是葡萄糖毒性的主要原因之一,但对该反应的位点特异性仍知之甚少。在此,采用了一种体外方法来详细研究人类全转铁蛋白糖基化。赖氨酸残基103、312和380被证明是最活泼的位点,并且发现总体糖基化特异性与载脂蛋白转铁蛋白所描述的显着不同。随后应用了一种计算生物化学方法来合理化赖氨酸反应性。即使p K一个值,溶剂可及表面积,氢键或附近的带电/极性残基的存在可能是相关的赖氨酸的反应性,这些参数不足以描述在全息转糖基化位点的特异性。此外,对最具反应性的残基的分析表明,正确的赖氨酸侧链取向可能在反应性中起重要作用。然而,在全运铁蛋白中,糖基化发生在铁结合位点以外,尽管观察到铁释放,但是载脂铁转铁蛋白的修饰对于在血清中观察到的铁结合能力的丧失应起更重要的作用。 DM患者。



























 京公网安备 11010802027423号
京公网安备 11010802027423号