当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Fragment-Based Discovery of a Regulatory Site in Thioredoxin Glutathione Reductase Acting as "Doorstop" for NADPH Entry.
ACS Chemical Biology ( IF 4 ) Pub Date : 2018-06-11 , DOI: 10.1021/acschembio.8b00349 Ilaria Silvestri 1 , Haining Lyu 2 , Francesca Fata 1 , Giovanna Boumis 3 , Adriana E Miele 3, 4 , Matteo Ardini 1 , Rodolfo Ippoliti 1 , Andrea Bellelli 3 , Ajit Jadhav 5 , Wendy A Lea 5 , Anton Simeonov 5 , Qing Cheng 6 , Elias S J Arnér 6 , Gregory R J Thatcher 7 , Pavel A Petukhov 7 , David L Williams 2 , Francesco Angelucci 1
ACS Chemical Biology ( IF 4 ) Pub Date : 2018-06-11 , DOI: 10.1021/acschembio.8b00349 Ilaria Silvestri 1 , Haining Lyu 2 , Francesca Fata 1 , Giovanna Boumis 3 , Adriana E Miele 3, 4 , Matteo Ardini 1 , Rodolfo Ippoliti 1 , Andrea Bellelli 3 , Ajit Jadhav 5 , Wendy A Lea 5 , Anton Simeonov 5 , Qing Cheng 6 , Elias S J Arnér 6 , Gregory R J Thatcher 7 , Pavel A Petukhov 7 , David L Williams 2 , Francesco Angelucci 1
Affiliation
|
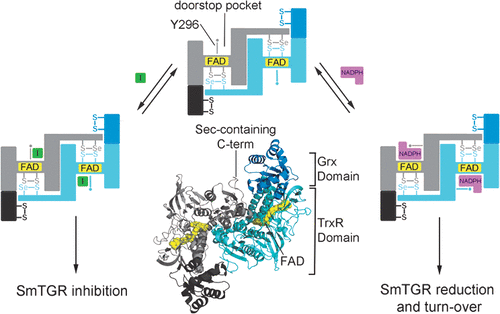
Members of the FAD/NAD-linked reductase family are recognized as crucial targets in drug development for cancers, inflammatory disorders, and infectious diseases. However, individual FAD/NAD reductases are difficult to inhibit in a selective manner with off-target inhibition reducing usefulness of identified compounds. Thioredoxin glutathione reductase (TGR), a high molecular weight thioredoxin reductase-like enzyme, has emerged as a promising drug target for the treatment of schistosomiasis, a parasitosis afflicting more than 200 million people. Taking advantage of small molecules selected from a high-throughput screen and using X-ray crystallography, functional assays, and docking studies, we identify a critical secondary site of the enzyme. Compounds binding at this site interfere with well-known and conserved conformational changes associated with NADPH reduction, acting as a doorstop for cofactor entry. They selectively inhibit TGR from Schistosoma mansoni and are active against parasites in culture. Since many members of the FAD/NAD-linked reductase family have similar catalytic mechanisms, the unique mechanism of inhibition identified in this study for TGR broadly opens new routes to selectively inhibit homologous enzymes of central importance in numerous diseases.
中文翻译:

基于片段的硫氧还蛋白谷胱甘肽还原酶调节位点的发现作为 NADPH 进入的“门挡”。
FAD/NAD 连接的还原酶家族的成员被认为是癌症、炎症性疾病和传染病药物开发的关键靶标。然而,单个 FAD/NAD 还原酶难以以选择性方式抑制,脱靶抑制会降低已鉴定化合物的有用性。硫氧还蛋白谷胱甘肽还原酶 (TGR) 是一种高分子量硫氧还蛋白还原酶样酶,已成为治疗血吸虫病的有希望的药物靶标,血吸虫病是一种折磨 2 亿多人的寄生虫病。利用从高通量筛选中选择的小分子,并使用 X 射线晶体学、功能测定和对接研究,我们确定了酶的关键次要位点。在此位点结合的化合物会干扰与 NADPH 减少相关的众所周知且保守的构象变化,充当辅助因子进入的门挡。它们选择性地抑制来自曼氏血吸虫的 TGR,并且对培养物中的寄生虫具有活性。由于 FAD/NAD 连接的还原酶家族的许多成员具有相似的催化机制,本研究中确定的 TGR 独特抑制机制广泛开辟了选择性抑制在众多疾病中具有核心重要性的同源酶的新途径。
更新日期:2018-05-25
中文翻译:

基于片段的硫氧还蛋白谷胱甘肽还原酶调节位点的发现作为 NADPH 进入的“门挡”。
FAD/NAD 连接的还原酶家族的成员被认为是癌症、炎症性疾病和传染病药物开发的关键靶标。然而,单个 FAD/NAD 还原酶难以以选择性方式抑制,脱靶抑制会降低已鉴定化合物的有用性。硫氧还蛋白谷胱甘肽还原酶 (TGR) 是一种高分子量硫氧还蛋白还原酶样酶,已成为治疗血吸虫病的有希望的药物靶标,血吸虫病是一种折磨 2 亿多人的寄生虫病。利用从高通量筛选中选择的小分子,并使用 X 射线晶体学、功能测定和对接研究,我们确定了酶的关键次要位点。在此位点结合的化合物会干扰与 NADPH 减少相关的众所周知且保守的构象变化,充当辅助因子进入的门挡。它们选择性地抑制来自曼氏血吸虫的 TGR,并且对培养物中的寄生虫具有活性。由于 FAD/NAD 连接的还原酶家族的许多成员具有相似的催化机制,本研究中确定的 TGR 独特抑制机制广泛开辟了选择性抑制在众多疾病中具有核心重要性的同源酶的新途径。



























 京公网安备 11010802027423号
京公网安备 11010802027423号