当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Control of Selectivity in Palladium(II)-Catalyzed Oxidative Transformations of Allenes
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2018-05-24 00:00:00 , DOI: 10.1021/acs.accounts.8b00138 Bin Yang 1 , Youai Qiu 1 , Jan-E. Bäckvall 1
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2018-05-24 00:00:00 , DOI: 10.1021/acs.accounts.8b00138 Bin Yang 1 , Youai Qiu 1 , Jan-E. Bäckvall 1
Affiliation
|
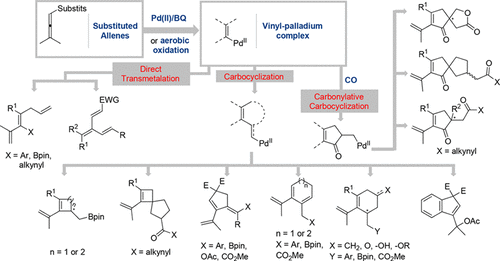
Oxidation reactions play a central role in organic synthesis, and it is highly desirable that these reactions are mild and occur under catalytic conditions. In Nature, oxidation reactions occur under mild conditions via cascade processes, and furthermore, they often occur in an enantioselective manner with many of them involving molecular oxygen or hydrogen peroxide as the terminal oxidant. Inspired by the reactions in Nature, we have developed a number of Pd(II)-catalyzed cascade reactions under mild oxidative conditions. These reactions have an intrinsic advantage of step economy and rely on selectivity control in each step. In this Account, we will discuss the control of chemo-, regio-, and diastereoselectivity in Pd(II)-catalyzed dehydrogenative cascade coupling reactions. The enantioselective version of this methodology has also been addressed, and new chiral centers have been introduced using a catalytic amount of a chiral phosphoric acid (CPA).
中文翻译:

钯(II)催化的丙二烯氧化转化选择性的控制
氧化反应在有机合成中起着核心作用,并且非常希望这些反应是温和的并且在催化条件下进行。在自然界中,氧化反应是在温和条件下通过级联过程发生的,此外,它们通常以对映选择性的方式发生,其中许多涉及分子氧或过氧化氢作为末端氧化剂。受自然界中反应的启发,我们开发了许多在轻度氧化条件下Pd(II)催化的级联反应。这些反应具有步骤经济的内在优势,并且在每个步骤中都依赖于选择性控制。在此帐户中,我们将讨论Pd(II)催化的脱氢级联反应中化学,区域和非对映选择性的控制。
更新日期:2018-05-24
中文翻译:

钯(II)催化的丙二烯氧化转化选择性的控制
氧化反应在有机合成中起着核心作用,并且非常希望这些反应是温和的并且在催化条件下进行。在自然界中,氧化反应是在温和条件下通过级联过程发生的,此外,它们通常以对映选择性的方式发生,其中许多涉及分子氧或过氧化氢作为末端氧化剂。受自然界中反应的启发,我们开发了许多在轻度氧化条件下Pd(II)催化的级联反应。这些反应具有步骤经济的内在优势,并且在每个步骤中都依赖于选择性控制。在此帐户中,我们将讨论Pd(II)催化的脱氢级联反应中化学,区域和非对映选择性的控制。



























 京公网安备 11010802027423号
京公网安备 11010802027423号