Chemical Physics Letters ( IF 2.8 ) Pub Date : 2018-05-24 , DOI: 10.1016/j.cplett.2018.05.045 Zongying Wang , Tianshui Liang , Yongpeng Yang , Xiangjian Shen
|
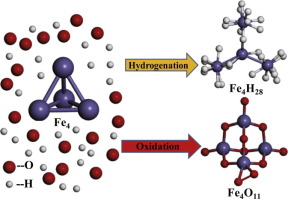
The interactions between atoms and small metal clusters are of great significance in the exploration of novel bonding mechanism. We present the strong chemisorption abilities of ultrasmall iron clusters interacting with large numbers of hydrogen and oxygen atoms from first-principles calculations. Geometric structures show that, the maximum numbers of the sequential hydrogen (oxygen) chemisorption on Fe1 to Fe4 clusters can be 11 to 28 (only 5 to 11). Using ab initio molecular dynamics, different kinds of hydrogen diffusion behaviors are found in the Fe-H systems, and two main types of Fe-O bonds are addressed in the stable Fe-O systems.
中文翻译:

氢和氧在超小型铁团簇上的高化学吸附能力:第一性原理研究
原子与小金属簇之间的相互作用对探索新型键合机理具有重要意义。从第一性原理计算中,我们展示了与大量氢和氧原子相互作用的超小铁簇的强大化学吸附能力。几何结构表明,Fe 1至Fe 4簇上的连续氢(氧)化学吸附的最大数目可以为11至28(仅5至11)。使用从头算的分子动力学,在Fe-H系统中发现了不同种类的氢扩散行为,并且在稳定的Fe-O系统中研究了两种主要类型的Fe-O键。



























 京公网安备 11010802027423号
京公网安备 11010802027423号