当前位置:
X-MOL 学术
›
Cell Chem. Bio.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
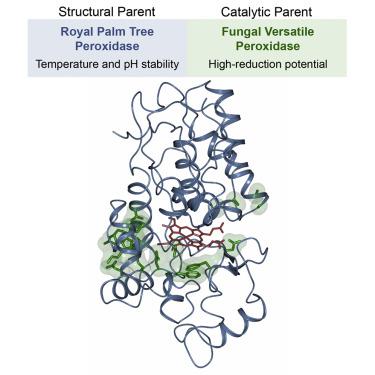
Structure-based Engineering of a Plant-Fungal Hybrid Peroxidase for Enhanced Temperature and pH Tolerance
Cell Chemical Biology ( IF 8.6 ) Pub Date : 2018-05-24 , DOI: 10.1016/j.chembiol.2018.04.014 Amanda C. Kohler , Blake A. Simmons , Kenneth L. Sale
Cell Chemical Biology ( IF 8.6 ) Pub Date : 2018-05-24 , DOI: 10.1016/j.chembiol.2018.04.014 Amanda C. Kohler , Blake A. Simmons , Kenneth L. Sale
|
In an age of ever-increasing biotechnological and industrial demand for new and specialized biocatalysts, rational protein engineering offers a direct approach to enzyme design and innovation. Heme peroxidases, as indispensable oxidative biocatalysts, provide a relatively mild alternative to the traditional harsh, and often toxic, chemical catalysts, and subsequently, have found widespread application throughout industry. However, the potential for these enzymes is far greater than their present use, as processes are currently restricted to the more stable, but less catalytically powerful, subset of peroxidases. Here we describe the structure-guided, rational engineering of a plant-fungal hybrid peroxidase built to overcome the application barrier of these high-reduction potential peroxidases. This engineered enzyme has the catalytic versatility and oxidative ability of a high-reduction potential versatile peroxidase, with enhanced temperature and pH tolerance similar to that of a highly stable plant peroxidase.
中文翻译:

植物-真菌杂化过氧化物酶的基于结构的工程,以提高温度和pH耐受性
在生物技术和工业对新型专用生物催化剂的需求不断增长的时代,合理的蛋白质工程为酶的设计和创新提供了直接的方法。血红素过氧化物酶作为必不可少的氧化生物催化剂,提供了相对温和的替代品,可替代传统的苛刻且通常有毒的化学催化剂,随后在整个工业中得到了广泛的应用。但是,这些酶的潜力远大于其目前的用途,因为目前的方法仅限于过氧化物酶的更稳定,但催化能力较弱的子集。在这里,我们描述了一种植物-真菌杂化过氧化物酶的结构指导性合理工程,旨在克服这些高还原电位过氧化物酶的应用障碍。
更新日期:2018-08-17
中文翻译:

植物-真菌杂化过氧化物酶的基于结构的工程,以提高温度和pH耐受性
在生物技术和工业对新型专用生物催化剂的需求不断增长的时代,合理的蛋白质工程为酶的设计和创新提供了直接的方法。血红素过氧化物酶作为必不可少的氧化生物催化剂,提供了相对温和的替代品,可替代传统的苛刻且通常有毒的化学催化剂,随后在整个工业中得到了广泛的应用。但是,这些酶的潜力远大于其目前的用途,因为目前的方法仅限于过氧化物酶的更稳定,但催化能力较弱的子集。在这里,我们描述了一种植物-真菌杂化过氧化物酶的结构指导性合理工程,旨在克服这些高还原电位过氧化物酶的应用障碍。



























 京公网安备 11010802027423号
京公网安备 11010802027423号