当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Enhancement of CO2 binding and mechanical properties upon diamine functionalization of M2(dobpdc) metal–organic frameworks†
Chemical Science ( IF 8.4 ) Pub Date : 2018-05-23 00:00:00 , DOI: 10.1039/c7sc05217k Jung-Hoon Lee 1, 2, 3, 4, 5 , Rebecca L. Siegelman 3, 4, 6, 7, 8 , Lorenzo Maserati 1, 2, 3, 4 , Tonatiuh Rangel 1, 2, 3, 4, 5 , Brett A. Helms 1, 2, 3, 4, 8 , Jeffrey R. Long 3, 4, 6, 7, 8 , Jeffrey B. Neaton 1, 2, 3, 4, 5
Chemical Science ( IF 8.4 ) Pub Date : 2018-05-23 00:00:00 , DOI: 10.1039/c7sc05217k Jung-Hoon Lee 1, 2, 3, 4, 5 , Rebecca L. Siegelman 3, 4, 6, 7, 8 , Lorenzo Maserati 1, 2, 3, 4 , Tonatiuh Rangel 1, 2, 3, 4, 5 , Brett A. Helms 1, 2, 3, 4, 8 , Jeffrey R. Long 3, 4, 6, 7, 8 , Jeffrey B. Neaton 1, 2, 3, 4, 5
Affiliation
|
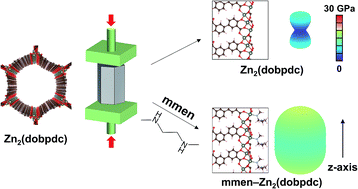
The family of diamine-appended metal–organic frameworks exemplified by compounds of the type mmen–M2(dobpdc) (mmen = N,N′-dimethylethylenediamine; M = Mg, Mn, Fe, Co, Zn; dobpdc4− = 4,4′-dioxidobiphenyl-3,3′-dicarboxylate) are adsorbents with significant potential for carbon capture, due to their high working capacities and strong selectivity for CO2 that stem from a cooperative adsorption mechanism. Herein, we use first-principles density functional theory (DFT) calculations to quantitatively investigate the role of mmen ligands in dictating the framework properties. Our van der Waals-corrected DFT calculations indicate that electrostatic interactions between ammonium carbamate units significantly enhance the CO2 binding strength relative to the unfunctionalized frameworks. Additionally, our computed energetics show that mmen–M2(dobpdc) materials can selectively adsorb CO2 under humid conditions, in agreement with experimental observations. The calculations further predict an increase of 112% and 124% in the orientationally-averaged Young's modulus E and shear modulus G, respectively, for mmen–Zn2(dobpdc) compared to Zn2(dobpdc), revealing a dramatic enhancement of mechanical properties associated with diamine functionalization. Taken together, our calculations demonstrate how functionalization with mmen ligands can enhance framework gas adsorption and mechanical properties.
中文翻译:

M 2(dobpdc)金属-有机骨架的二胺官能化后 ,CO 2结合力和机械性能的增强†
带有二胺的金属有机骨架家族,以mmen-M 2(dobpdc)类型的化合物为例(mmen = N,N'-二甲基乙二胺; M = Mg,Mn,Fe,Co,Zn; dobpdc 4- = 4 (4'-dioxidobiphenyl-3,3'-dicarboxylate)是吸附剂,由于其高工作能力和对CO 2的强选择性而具有很强的碳捕获潜力。这源于协同吸附机制。在这里,我们使用第一原理密度泛函理论(DFT)计算来定量研究mmen配体在决定骨架性质中的作用。我们的范德华校正DFT计算表明,相对于未官能化的骨架,氨基甲酸铵单元之间的静电相互作用显着增强了CO 2的结合强度。另外,我们的计算的能量学表明,mmen–M 2(dobpdc)材料可以在潮湿条件下选择性吸附CO 2,这与实验观察一致。该计算进一步预测了取向平均杨氏模量E和剪切模量的112%和124%的增加ģ,分别用于mmen-Zn系2(dobpdc)相比对于Zn 2(dobpdc),揭示了用二胺官能化相关的机械性能的显着提高。两者合计,我们的计算证明了mmen配体的官能化如何增强骨架气体的吸附和机械性能。
更新日期:2018-05-23
中文翻译:

M 2(dobpdc)金属-有机骨架的二胺官能化后 ,CO 2结合力和机械性能的增强†
带有二胺的金属有机骨架家族,以mmen-M 2(dobpdc)类型的化合物为例(mmen = N,N'-二甲基乙二胺; M = Mg,Mn,Fe,Co,Zn; dobpdc 4- = 4 (4'-dioxidobiphenyl-3,3'-dicarboxylate)是吸附剂,由于其高工作能力和对CO 2的强选择性而具有很强的碳捕获潜力。这源于协同吸附机制。在这里,我们使用第一原理密度泛函理论(DFT)计算来定量研究mmen配体在决定骨架性质中的作用。我们的范德华校正DFT计算表明,相对于未官能化的骨架,氨基甲酸铵单元之间的静电相互作用显着增强了CO 2的结合强度。另外,我们的计算的能量学表明,mmen–M 2(dobpdc)材料可以在潮湿条件下选择性吸附CO 2,这与实验观察一致。该计算进一步预测了取向平均杨氏模量E和剪切模量的112%和124%的增加ģ,分别用于mmen-Zn系2(dobpdc)相比对于Zn 2(dobpdc),揭示了用二胺官能化相关的机械性能的显着提高。两者合计,我们的计算证明了mmen配体的官能化如何增强骨架气体的吸附和机械性能。


























 京公网安备 11010802027423号
京公网安备 11010802027423号