Bioorganic Chemistry ( IF 5.1 ) Pub Date : 2018-05-23 , DOI: 10.1016/j.bioorg.2018.05.017 Basharat Ali , Khalid Mohammed Khan , Uzma Salar , Kanwal , Safdar Hussain , Muhammad Ashraf , Muhammad Riaz , Abdul Wadood , Muhammad Taha , Shahnaz Perveen
|
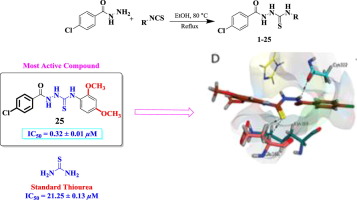
A series of 1-[(4′-chlorophenyl)carbonyl-4-(aryl)thiosemicarbazide derivatives 1–25 was synthesized and characterized by spectroscopic techniques such as EI-MS and 1H NMR. All compounds were screened for urease inhibitory activity in vitro and demonstrated excellent inhibitory activity in the range of IC50 = 0.32 ± 0.01–25.13 ± 0.13 μM as compared to the standard thiourea (IC50 = 21.25 ± 0.13 μM). Amongst the potent analogs, compounds 3 (IC50 = 2.31 ± 0.01 μM), 6 (IC50 = 2.14 ± 0.04 μM), 10 (IC50 = 1.14 ± 0.06 μM), 20 (IC50 = 2.15 ± 0.05 μM), and 25 (IC50 = 0.32 ± 0.01 μM) are many folds more active than the standard. Structure-activity relationship (SAR) was rationalized by looking at the effect of diversely substituted aryl ring on inhibitory potential which predicted that regardless of the nature of substituents, their positions on aryl ring is worth important for the potent activity. Furthermore, to verify these interpretations, in silico study was performed on all compounds and a good correlation was perceived between the biological evaluation and docking study of compounds.
中文翻译:

1-[(4'-氯苯基)羰基-4-(芳基)硫代氨基脲衍生物作为有效的脲酶抑制剂:合成,体外和计算机研究
合成了一系列1-[((4'-氯苯基)羰基-4-(芳基)硫代氨基脲] 1 – 25,并通过光谱技术(例如EI-MS和1 H NMR)进行了表征。 与标准硫脲(IC 50 = 21.25± 0.13μM)相比,所有化合物的体外尿素酶抑制活性均得到筛选,并在IC 50 = 0.32±0.01–25.13±0.13μM范围内表现出优异的抑制活性。在有效的类似物中,化合物3(IC 50 = 2.31±0.01μM),6(IC 50 = 2.14±0.04μM),10(IC 50 = 1.14±0.06μM),20(IC50 = 2.15±0.05μM)和25(IC 50 = 0.32±0.01μM)的活性比标准品高很多倍。通过考察不同取代的芳基环对抑制电位的影响来合理化结构-活性关系(SAR),该效应预测,不管取代基的性质如何,它们在芳基环上的位置对于有效活性都很重要。此外,为验证这些解释,对所有化合物进行了计算机分析,并在生物学评估和化合物的对接研究之间建立了良好的相关性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号