当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
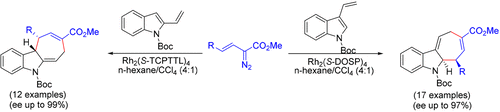
Rhodium-Catalyzed Asymmetric Dearomative [4 + 3]-Cycloaddition of Vinylindoles with Vinyldiazoacetates: Access to Cyclohepta[b]indoles
Organic Letters ( IF 5.2 ) Pub Date : 2018-05-23 00:00:00 , DOI: 10.1021/acs.orglett.8b01353 Guangyang Xu 1 , Long Chen 1 , Jiangtao Sun 1
Organic Letters ( IF 5.2 ) Pub Date : 2018-05-23 00:00:00 , DOI: 10.1021/acs.orglett.8b01353 Guangyang Xu 1 , Long Chen 1 , Jiangtao Sun 1
Affiliation
|
A rhodium-catalyzed enantioselective formal [4 + 3]-cycloaddition of vinylindoles with vinyldiazoacetates has been developed, affording the dearomative cyclization products containing a newly formed seven-membered ring in up to 99% ee. Rh2(S-DOSP)4 has been proven to be the best catalyst for the cycloaddition of 3-vinylindoles with vinyldiazoacetates, whereas Rh2(S-TCPTTL)4 has enhanced the enantioselectivity for 2-vinylindoles.
中文翻译:

铑催化的乙烯基吲哚乙酸与乙烯基吲哚的不对称脱芳香性[4 + 3]-环加成反应:获得环庚基[ b ]吲哚
乙烯基吲哚与乙烯基重氮乙酸酯的铑催化的对映体选择性[4 + 3]-环加成反应已经开发出来,提供了脱芳香环化产物,该产物含有高达99%ee的新形成的七元环。Rh 2(S -DOSP)4被证明是3-乙烯基吲哚与乙烯基重氮乙酸酯环加成的最佳催化剂,而Rh 2(S -TCPTTL)4增强了2-乙烯基吲哚的对映选择性。
更新日期:2018-05-23
中文翻译:

铑催化的乙烯基吲哚乙酸与乙烯基吲哚的不对称脱芳香性[4 + 3]-环加成反应:获得环庚基[ b ]吲哚
乙烯基吲哚与乙烯基重氮乙酸酯的铑催化的对映体选择性[4 + 3]-环加成反应已经开发出来,提供了脱芳香环化产物,该产物含有高达99%ee的新形成的七元环。Rh 2(S -DOSP)4被证明是3-乙烯基吲哚与乙烯基重氮乙酸酯环加成的最佳催化剂,而Rh 2(S -TCPTTL)4增强了2-乙烯基吲哚的对映选择性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号