当前位置:
X-MOL 学术
›
Biomater. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synergistic breast tumor cell killing achieved by intracellular co-delivery of doxorubicin and disulfiram via core–shell–corona nanoparticles†
Biomaterials Science ( IF 6.6 ) Pub Date : 2018-05-22 00:00:00 , DOI: 10.1039/c8bm00271a Xiaoguang Tao 1, 2, 3, 4, 5 , Jingxin Gou 1, 2, 3, 4, 5 , Qianying Zhang 1, 2, 3, 4, 5 , Xinyi Tan 1, 2, 3, 4, 5 , Tianyang Ren 1, 2, 3, 4, 5 , Qing Yao 1, 2, 3, 4, 5 , Bin Tian 5, 6, 7, 8 , Longfa Kou 1, 2, 3, 4, 5 , Ling Zhang 4, 5, 9, 10, 11 , Xing Tang 1, 2, 3, 4, 5
Biomaterials Science ( IF 6.6 ) Pub Date : 2018-05-22 00:00:00 , DOI: 10.1039/c8bm00271a Xiaoguang Tao 1, 2, 3, 4, 5 , Jingxin Gou 1, 2, 3, 4, 5 , Qianying Zhang 1, 2, 3, 4, 5 , Xinyi Tan 1, 2, 3, 4, 5 , Tianyang Ren 1, 2, 3, 4, 5 , Qing Yao 1, 2, 3, 4, 5 , Bin Tian 5, 6, 7, 8 , Longfa Kou 1, 2, 3, 4, 5 , Ling Zhang 4, 5, 9, 10, 11 , Xing Tang 1, 2, 3, 4, 5
Affiliation
|
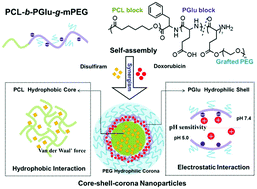
Combination therapy with different functional chemotherapeutic agents based on nano-drug delivery systems is an effective strategy for the treatment of breast cancer. However, co-delivery of drug molecules with different physicochemical properties still remains a challenge. In this study, an amphiphilic poly (ε-caprolactone)-b-poly (L-glutamic acid)-g-methoxy poly (ethylene glycol) (PCL-b-PGlu-g-mPEG) copolymer was designed and synthesized to develop a nanocarrier for the co-delivery of hydrophilic doxorubicin (DOX) and hydrophobic disulfiram (DSF). The amphiphilic copolymer self-assembled into core–shell–corona structured nanoparticles with the hydrophobic PCL core for DSF loading (hydrophobic interaction) and anionic poly (glutamic acid) shell for DOX loading (electrostatic interaction). DSF and DOX co-loaded nanoparticles (Co-NPs) resulted in high drug loading and precisely controlled DSF/DOX ratio via formulation optimization. Compared with free drug solutions, DSF and DOX delivered by the Co-NPs were found to have improved intracellular accumulation. Results of cytotoxicity assays showed that DSF/DOX delivered at the weight ratio of 0.5 and 1 could achieve a synergistic cytotoxic effect on breast cancer cell lines (MCF-7 and MDA-MB-231). In vivo imaging confirmed that the core–shell–corona nanoparticles could efficiently accumulate in tumors. In vivo anti-tumor effect results indicated that Co-NPs showed an improved drug synergistic effect on antitumor activity compared with the free drug combination. Therefore, it can be concluded that core–shell–corona nanoparticles prepared by PCL-b-PGlu-g-mPEG could be a promising co-delivery system for drug combination therapy in the treatment of breast cancer.
中文翻译:

协同乳腺肿瘤细胞杀伤由多柔比星和双硫仑的细胞内共递送实现经由芯壳纳米粒子的电晕†
基于纳米药物递送系统与不同功能化学治疗剂的联合治疗是治疗乳腺癌的有效策略。然而,具有不同物理化学性质的药物分子的共同递送仍然是一个挑战。在这项研究中,两亲性聚(ε-己内酯)-b-聚(L-谷氨酸)-g-甲氧基聚(乙二醇)(PCL- b- PGlu- g-mPEG)共聚物经过设计和合成,以开发用于亲水性阿霉素(DOX)和疏水性双硫仑(DSF)共递送的纳米载体。两亲共聚物自组装为核-壳-电晕结构的纳米粒子,具有疏水的PCL核用于DSF加载(疏水相互作用)和阴离子聚(谷氨酸)壳用于DOX加载(静电相互作用)。DSF和DOX共纳米粒(联合NPS)导致高载药量和精确控制的DSF / DOX比通过配方优化。与游离药物溶液相比,发现由Co-NP传递的DSF和DOX具有改善的细胞内积累。细胞毒性试验的结果表明,以0.5和1的重量比递送的DSF / DOX对乳腺癌细胞系(MCF-7和MDA-MB-231)具有协同的细胞毒性作用。体内成像证实,核-壳-电晕纳米颗粒可以有效地在肿瘤中蓄积。体内抗肿瘤作用结果表明,与游离药物组合相比,Co-NPs对抗肿瘤活性显示出改善的药物协同作用。因此,可以得出结论,PCL - b- PGlu- g制备的核-壳-电晕纳米颗粒-mPEG可能是治疗乳腺癌的药物联合治疗的有希望的共同给药系统。
更新日期:2018-05-22
中文翻译:

协同乳腺肿瘤细胞杀伤由多柔比星和双硫仑的细胞内共递送实现经由芯壳纳米粒子的电晕†
基于纳米药物递送系统与不同功能化学治疗剂的联合治疗是治疗乳腺癌的有效策略。然而,具有不同物理化学性质的药物分子的共同递送仍然是一个挑战。在这项研究中,两亲性聚(ε-己内酯)-b-聚(L-谷氨酸)-g-甲氧基聚(乙二醇)(PCL- b- PGlu- g-mPEG)共聚物经过设计和合成,以开发用于亲水性阿霉素(DOX)和疏水性双硫仑(DSF)共递送的纳米载体。两亲共聚物自组装为核-壳-电晕结构的纳米粒子,具有疏水的PCL核用于DSF加载(疏水相互作用)和阴离子聚(谷氨酸)壳用于DOX加载(静电相互作用)。DSF和DOX共纳米粒(联合NPS)导致高载药量和精确控制的DSF / DOX比通过配方优化。与游离药物溶液相比,发现由Co-NP传递的DSF和DOX具有改善的细胞内积累。细胞毒性试验的结果表明,以0.5和1的重量比递送的DSF / DOX对乳腺癌细胞系(MCF-7和MDA-MB-231)具有协同的细胞毒性作用。体内成像证实,核-壳-电晕纳米颗粒可以有效地在肿瘤中蓄积。体内抗肿瘤作用结果表明,与游离药物组合相比,Co-NPs对抗肿瘤活性显示出改善的药物协同作用。因此,可以得出结论,PCL - b- PGlu- g制备的核-壳-电晕纳米颗粒-mPEG可能是治疗乳腺癌的药物联合治疗的有希望的共同给药系统。


























 京公网安备 11010802027423号
京公网安备 11010802027423号