当前位置:
X-MOL 学术
›
J. Mater. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
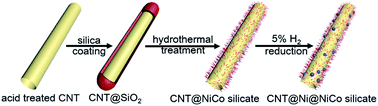
CNT@Ni@Ni–Co silicate core–shell nanocomposite: a synergistic triple-coaxial catalyst for enhancing catalytic activity and controlling side products for Li–O2 batteries†
Journal of Materials Chemistry A ( IF 11.9 ) Pub Date : 2018-05-21 00:00:00 , DOI: 10.1039/c8ta03099e Ziwei Li 1, 2, 3, 4, 5 , Junghoon Yang 1, 2, 3, 4 , Daniel Adjei Agyeman 1, 2, 3, 4 , Mihui Park 1, 2, 3, 4 , Wilson Tamakloe 1, 2, 3, 4 , Yusuke Yamauchi 5, 6, 7, 8, 9 , Yong-Mook Kang 1, 2, 3, 4
Journal of Materials Chemistry A ( IF 11.9 ) Pub Date : 2018-05-21 00:00:00 , DOI: 10.1039/c8ta03099e Ziwei Li 1, 2, 3, 4, 5 , Junghoon Yang 1, 2, 3, 4 , Daniel Adjei Agyeman 1, 2, 3, 4 , Mihui Park 1, 2, 3, 4 , Wilson Tamakloe 1, 2, 3, 4 , Yusuke Yamauchi 5, 6, 7, 8, 9 , Yong-Mook Kang 1, 2, 3, 4
Affiliation
|
A great challenge in the application of carbon-based materials to Li–O2 batteries is to prevent the formation of carbonate-based side products, thereby extending the cycle life of Li–O2 batteries. Herein, for the first time, CNT@Ni@NiCo silicate core–shell nanocomposite is designed and used as a cathode catalyst in Li–O2 batteries. This nanocomposite shows a promising electrochemical performance with a discharge capacity of 10 046 mA h gcat−1 and a low overpotential of 1.44 V at a current density of 200 mA gcat−1, and it can sustain for more than 50 cycles within the voltage range of 2–4.7 V. X-ray photoelectron spectroscopy (XPS) and X-ray diffraction (XRD) characterizations prove that the formation of Li2CO3 and other side products are prevented, likely due to the encapsulation of CNTs by NiCo silicates and Ni nanoparticles, which may help decompose the side products. Finally, the synergistic effects, which are contributed by the high electrical conductivity of CNTs, high surface area, the high oxygen reduction reaction (ORR) and oxygen evolution reaction (OER) activities of NiCo silicate, and the simple decomposition of side products by Ni nanoparticles enable outstanding performance of the CNT@Ni@NiCo silicate core–shell nanocomposite as a cathode catalyst for Li–O2 batteries.
中文翻译:

CNT @ Ni @ Ni–Co硅酸盐核壳纳米复合材料:协同三同轴催化剂,可增强Li–O 2电池的催化活性并控制副产物†
将碳基材料应用于Li-O 2电池的一个巨大挑战是防止形成碳酸盐基副产品,从而延长Li-O 2电池的循环寿命。在此,首次设计了CNT @ Ni @ NiCo硅酸盐核壳纳米复合材料,并将其用作Li-O 2电池的阴极催化剂。该纳米复合材料显示出令人鼓舞的电化学性能,在200 mA g cat -1的电流密度下的放电容量为10 046 mA hg cat -1,低过电位为1.44 V,并且可以在2–4.7 V的电压范围内维持超过50个循环。X射线光电子能谱(XPS)和X射线衍射(XRD)表征证明了Li 2 CO 3和其他副产物的形成。可能是由于NiCo硅酸盐和Ni纳米颗粒对CNT的封装所致,因此可能会导致副产物分解。最后,协同作用是由CNT的高电导率,高表面积,NiCo硅酸盐的高氧还原反应(ORR)和氧释放反应(OER)活性以及Ni副产物的简单分解所引起的。纳米粒子使CNT @ Ni @ NiCo硅酸盐核壳纳米复合材料具有出色的性能,可作为Li–O 2的阴极催化剂 电池。
更新日期:2018-05-21
中文翻译:

CNT @ Ni @ Ni–Co硅酸盐核壳纳米复合材料:协同三同轴催化剂,可增强Li–O 2电池的催化活性并控制副产物†
将碳基材料应用于Li-O 2电池的一个巨大挑战是防止形成碳酸盐基副产品,从而延长Li-O 2电池的循环寿命。在此,首次设计了CNT @ Ni @ NiCo硅酸盐核壳纳米复合材料,并将其用作Li-O 2电池的阴极催化剂。该纳米复合材料显示出令人鼓舞的电化学性能,在200 mA g cat -1的电流密度下的放电容量为10 046 mA hg cat -1,低过电位为1.44 V,并且可以在2–4.7 V的电压范围内维持超过50个循环。X射线光电子能谱(XPS)和X射线衍射(XRD)表征证明了Li 2 CO 3和其他副产物的形成。可能是由于NiCo硅酸盐和Ni纳米颗粒对CNT的封装所致,因此可能会导致副产物分解。最后,协同作用是由CNT的高电导率,高表面积,NiCo硅酸盐的高氧还原反应(ORR)和氧释放反应(OER)活性以及Ni副产物的简单分解所引起的。纳米粒子使CNT @ Ni @ NiCo硅酸盐核壳纳米复合材料具有出色的性能,可作为Li–O 2的阴极催化剂 电池。



























 京公网安备 11010802027423号
京公网安备 11010802027423号