Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Characterization of the Ionic Liquid/Electrode Interfacial Relaxation Processes Under Potential Polarization for Ionic Liquid Amperometric Gas Sensor Method Development.
ACS Sensors ( IF 8.9 ) Pub Date : 2018-06-04 , DOI: 10.1021/acssensors.8b00155 Lu Lin , Peng Zhao , Andrew J Mason 1 , Xiangqun Zeng
ACS Sensors ( IF 8.9 ) Pub Date : 2018-06-04 , DOI: 10.1021/acssensors.8b00155 Lu Lin , Peng Zhao , Andrew J Mason 1 , Xiangqun Zeng
Affiliation
|
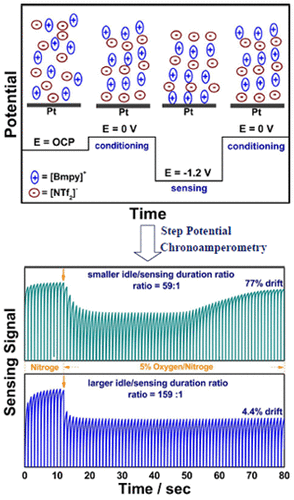
Electrochemical amperometric sensors require a constant or varying potential at the working electrode that drives redox reactions of the analyte for detection. The interfacial redox reaction(s) can result in the formation of new chemical products that could change the initial condition of the electrode/electrolyte interface. If the products are not inert and/or cannot be removed from the system such that the initial condition of the electrode/electrolyte interface cannot be restored, the sensor signal baseline would consequently drift, which is problematic for the continuous and real-time sensors. By setting the electrode potential with the periodical ON-OFF mode, electrolysis can be forestalled during the off mode which can minimize the sensor signal baseline drift and reduce the power consumption of the sensor. However, it is known that the relaxation of the structure in the electrical double layer at the ionic liquid/electrode interface to the steps of the electrode potential is slow. This work characterized the electrode/electrolyte interfacial relaxation process of an ionic liquid based electrochemical gas (IL-EG) sensor by performing multiple potential step experiments in which the potential is stepped from an open circuit potential (OCP) to the amperometric sensing potential at various frequencies with different time periods. Our results showed that by shortening the sensing period as well as extending the idle period (i.e., enlarge the ratio of idle period versus sensing period) of the potential step experiments, the electrode/electrolyte interface is prone to relax to its original state, and thus reduces the baseline drift. Additionally, the high viscosity of the ionic liquids is beneficial for electrochemical regeneration via the implementation of a conditioning step at zero volts at the electrode/electrolyte. By setting the working electrode at zero volts instead of OCP, our results showed that it could further minimize the baseline drift, enhance the sensing signal stability, and extend the functioning lifetime of a continuous IL-EG oxygen sensor.
中文翻译:

离子液体/电极界面弛豫过程在电位极化下的表征,用于离子液体安培气体传感器方法的开发。
电化学安培传感器需要在工作电极上保持恒定或变化的电势,以驱动分析物的氧化还原反应进行检测。界面氧化还原反应可导致形成新的化学产物,这些化学产物可改变电极/电解质界面的初始条件。如果产物不是惰性的和/或不能从系统中除去,使得电极/电解质界面的初始状态无法恢复,则传感器信号基线将因此漂移,这对于连续和实时传感器而言是成问题的。通过使用周期性的ON-OFF模式设置电极电势,可以在关闭模式下阻止电解,这可以最大程度地减少传感器信号基线漂移并降低传感器的功耗。然而,众所周知,在离子液体/电极界面处的双电层中的结构松弛到电极电势的台阶是缓慢的。这项工作通过执行多个电势阶跃实验来表征离子液体基电化学气体(IL-EG)传感器的电极/电解质界面弛豫过程,其中,电势从开路电势(OCP)跃迁到各种电流下的安培感测电势不同时间段的频率。我们的结果表明,通过缩短潜在步骤实验的感测周期并延长空闲周期(即,增大空闲周期与感应周期的比值),电极/电解质界面易于松弛至其原始状态,并且因此减少了基线漂移。此外,离子液体的高粘度通过在电极/电解质上以零伏的电压进行调节步骤,有利于电化学再生。通过将工作电极设置为零伏而不是OCP,我们的结果表明,它可以进一步最小化基线漂移,增强传感信号的稳定性,并延长连续IL-EG氧气传感器的使用寿命。
更新日期:2018-05-21
中文翻译:

离子液体/电极界面弛豫过程在电位极化下的表征,用于离子液体安培气体传感器方法的开发。
电化学安培传感器需要在工作电极上保持恒定或变化的电势,以驱动分析物的氧化还原反应进行检测。界面氧化还原反应可导致形成新的化学产物,这些化学产物可改变电极/电解质界面的初始条件。如果产物不是惰性的和/或不能从系统中除去,使得电极/电解质界面的初始状态无法恢复,则传感器信号基线将因此漂移,这对于连续和实时传感器而言是成问题的。通过使用周期性的ON-OFF模式设置电极电势,可以在关闭模式下阻止电解,这可以最大程度地减少传感器信号基线漂移并降低传感器的功耗。然而,众所周知,在离子液体/电极界面处的双电层中的结构松弛到电极电势的台阶是缓慢的。这项工作通过执行多个电势阶跃实验来表征离子液体基电化学气体(IL-EG)传感器的电极/电解质界面弛豫过程,其中,电势从开路电势(OCP)跃迁到各种电流下的安培感测电势不同时间段的频率。我们的结果表明,通过缩短潜在步骤实验的感测周期并延长空闲周期(即,增大空闲周期与感应周期的比值),电极/电解质界面易于松弛至其原始状态,并且因此减少了基线漂移。此外,离子液体的高粘度通过在电极/电解质上以零伏的电压进行调节步骤,有利于电化学再生。通过将工作电极设置为零伏而不是OCP,我们的结果表明,它可以进一步最小化基线漂移,增强传感信号的稳定性,并延长连续IL-EG氧气传感器的使用寿命。



























 京公网安备 11010802027423号
京公网安备 11010802027423号