当前位置:
X-MOL 学术
›
ChemMedChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis and Evaluation of a Series of Bis(pentylpyridinium) Compounds as Antifungal Agents
ChemMedChem ( IF 3.4 ) Pub Date : 2018-06-19 , DOI: 10.1002/cmdc.201800331 Daniel Obando 1 , Yasuko Koda 1, 2 , Namfon Pantarat 2 , Sophie Lev 2 , Xiaoming Zuo 2 , Johanes Bijosono Oei 2 , Fred Widmer 2 , Julianne T. Djordjevic 2 , Tania C. Sorrell 2 , Katrina A. Jolliffe 1
ChemMedChem ( IF 3.4 ) Pub Date : 2018-06-19 , DOI: 10.1002/cmdc.201800331 Daniel Obando 1 , Yasuko Koda 1, 2 , Namfon Pantarat 2 , Sophie Lev 2 , Xiaoming Zuo 2 , Johanes Bijosono Oei 2 , Fred Widmer 2 , Julianne T. Djordjevic 2 , Tania C. Sorrell 2 , Katrina A. Jolliffe 1
Affiliation
|
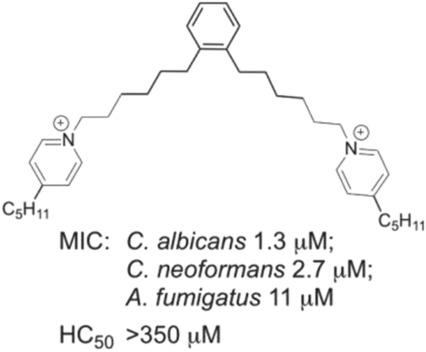
A series of bis(4‐pentylpyridinium) compounds with a variety of spacers between the pyridinium headgroups was synthesised, and the antifungal activity of these compounds was investigated. Lengthening the alkyl spacer between the pentylpyridinium headgroups from 12 to 16 methylene units resulted in increased antifungal activity against C. neoformans and C. albicans, but also resulted in increased hemolytic activity and cytotoxicity against mammalian cells. However, inclusion of an ortho‐substituted benzene ring in the centre of the alkyl spacer resulted in decreased cytotoxicity and hemolytic activity, while maintaining antifungal potency. Replacement of the alkyl and aromatic‐containing spacers by more hydrophilic ethylene glycol groups resulted in a loss of antifungal activity. Some of the compounds inhibited fungal PLB1 activity, but the low correlation of this inhibition with antifungal potency indicates PLB1 inhibition is unlikely to be the predominant mode of antifungal action of this class of compounds, with preliminary studies suggesting they may act via disruption of fungal mitochondrial function.
中文翻译:

一系列双(戊基吡啶)类化合物作为抗真菌剂的合成与评价
合成了一系列在吡啶鎓头基之间具有多个间隔基的双(4-戊基吡啶)化合物,并研究了这些化合物的抗真菌活性。从12至16个亚甲基单元延长pentylpyridinium头部基团之间的烷基间隔造成了对增加的抗真菌活性隐球菌和白色念珠菌,但也造成了对哺乳动物细胞中增加的溶血活性和细胞毒性。但是,包含邻位烷基间隔基中心的预取代苯环导致细胞毒性和溶血活性降低,同时保持了抗真菌效力。用亲水性更高的乙二醇取代烷基和芳香族间隔基会导致抗真菌活性下降。一些化合物抑制了真菌PLB1的活性,但这种抑制作用与抗真菌效力的低相关性表明PLB1抑制不太可能是此类化合物的主要抗真菌作用方式,初步研究表明它们可能通过破坏真菌线粒体发挥作用。功能。
更新日期:2018-06-19
中文翻译:

一系列双(戊基吡啶)类化合物作为抗真菌剂的合成与评价
合成了一系列在吡啶鎓头基之间具有多个间隔基的双(4-戊基吡啶)化合物,并研究了这些化合物的抗真菌活性。从12至16个亚甲基单元延长pentylpyridinium头部基团之间的烷基间隔造成了对增加的抗真菌活性隐球菌和白色念珠菌,但也造成了对哺乳动物细胞中增加的溶血活性和细胞毒性。但是,包含邻位烷基间隔基中心的预取代苯环导致细胞毒性和溶血活性降低,同时保持了抗真菌效力。用亲水性更高的乙二醇取代烷基和芳香族间隔基会导致抗真菌活性下降。一些化合物抑制了真菌PLB1的活性,但这种抑制作用与抗真菌效力的低相关性表明PLB1抑制不太可能是此类化合物的主要抗真菌作用方式,初步研究表明它们可能通过破坏真菌线粒体发挥作用。功能。



























 京公网安备 11010802027423号
京公网安备 11010802027423号