Journal of Inorganic Biochemistry ( IF 3.9 ) Pub Date : 2018-05-21 , DOI: 10.1016/j.jinorgbio.2018.05.010 Rami Masamrekh , Alexey Kuzikov , Alexander Veselovsky , Iliya Toropygin , Tatsiana Shkel , Natalia Strushkevich , Andrei Gilep , Sergey Usanov , Alexander Archakov , Victoria Shumyantseva
|
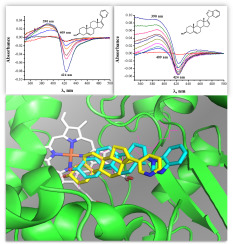
Abiraterone and galeterone induce type I differential spectral changes in human sterol 14α-demethylase (cytochrome P450 51A1, CYP51A1) with the sigmoidal shape of the binding curve. After approximation of the data by Hill model, the half-saturation concentrations (K0.5) were estimated as 22 ± 1 μM and 16 ± 1 μM and the Hill coefficients as 2.4 ± 0.2 and 1.97 ± 0.23 for abiraterone and galeterone, respectively. We analyzed the catalytic activity of CYP51A1 towards abiraterone and galeterone using an electrochemical system based on recombinant CYP51A1 immobilized on the screen-printed graphite electrode (SPE) modified by didodecyldimethylammonium bromide (DDAB) film. The study revealed the amperometric response of CYP51A1 upon addition of abiraterone, which may indicate the substrate properties of abiraterone towards CYP51A1. Galeterone caused negligible amperometric response of CYP51A1. Mass-spectrometric analysis of the products of CYP51A1-dependent electrocatalytic reaction at a controlled potential towards abiraterone and galeterone revealed products with m/z of 366.3 and 405.2, respectively, indicating monohydroxylation of abiraterone and galeterone. We have observed the sigmoidal character of the dependence of the catalytic current on abiraterone concentration. Analysis of molecular docking data demonstrated the ability of abiraterone and galeterone to bind to the active site of CYP51A1, but abiraterone occupies the position closer to the heme.
中文翻译:

17α-羟化酶,17(20)-裂合酶(CYP17A1)抑制剂阿比特龙和galeterone与人固醇14α-脱甲基酶(CYP51A1)的相互作用
Abiraterone和galeterone诱导人固醇14α-脱甲基酶(细胞色素P450 51A1,CYP51A1)具有结合曲线的S形形状的I型差异光谱变化。用希尔模型将数据近似后,半饱和浓度(K 0.5)估计阿比特龙和galeterone的)分别为22±1μM和16±1μM,Hill系数分别为2.4±0.2和1.97±0.23。我们使用基于重组CYP51A1的电化学系统,分析了CYP51A1对阿比特龙和galeterone的催化活性,该电化学系统基于固定在由十二烷基二甲基溴化铵(DDAB)膜修饰的丝网印刷石墨电极(SPE)上的重组CYP51A1。该研究揭示了添加阿比特龙后CYP51A1的安培响应,这可能表明阿比特龙对CYP51A1的底物性质。Galeterone对CYP51A1的安培反应的影响可忽略不计。CYP51A1依赖的电催化反应产物在受控电位下对阿比特龙和加利泰隆的质谱分析显示产物具有m / z分别为366.3和405.2,表明阿比特龙和加勒酮的单羟基化。我们已经观察到催化电流对阿比特龙浓度的依赖性的S形特征。分子对接数据的分析证明了阿比特龙和加利泰隆具有与CYP51A1活性位点结合的能力,但阿比特龙占据了更靠近血红素的位置。



























 京公网安备 11010802027423号
京公网安备 11010802027423号