当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Total Synthesis of Laingolide B Stereoisomers and Assignment of Absolute Configuration
Organic Letters ( IF 5.2 ) Pub Date : 2018-05-21 00:00:00 , DOI: 10.1021/acs.orglett.8b01269 Chengsen Cui 1 , Wei-Min Dai 1
Organic Letters ( IF 5.2 ) Pub Date : 2018-05-21 00:00:00 , DOI: 10.1021/acs.orglett.8b01269 Chengsen Cui 1 , Wei-Min Dai 1
Affiliation
|
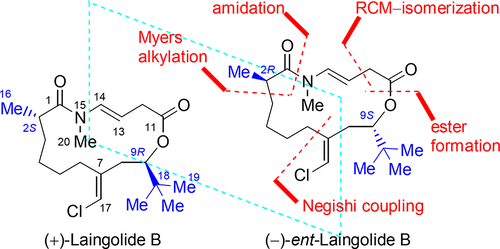
Total synthesis of (−)-(2R,9S)- and (+)-(2S,9S)-stereoisomers of laingolide B has been accomplished by using sequential ring-closing metathesis (RCM) and alkene isomerization to construct the macrocyclic trans-N-methyl enamide moiety. The Myers alkylation was used to secure the C2 stereochemistry of the two RCM precursors from a common (9S)-C3–C9 alkyl iodide. The absolute configuration of laingolide B has been assigned as (2S,9R) by comparison of the optical rotation data.
中文翻译:

Laingolide B立体异构体的全合成和绝对构型分配
Laingolide B的(-)-(2 R,9 S)-和(+)-(2 S,9 S)-立体异构体的全合成已通过使用顺序闭环复分解(RCM)和烯烃异构化来构建大环反式- ñ -甲基烯酰胺基团。Myers烷基化用于确保两种RCM前体的C2立体化学来自共同的(9 S)-C3-C9烷基碘。通过旋光度数据的比较,Laingolide B的绝对构型已指定为(2 S,9 R)。
更新日期:2018-05-21
中文翻译:

Laingolide B立体异构体的全合成和绝对构型分配
Laingolide B的(-)-(2 R,9 S)-和(+)-(2 S,9 S)-立体异构体的全合成已通过使用顺序闭环复分解(RCM)和烯烃异构化来构建大环反式- ñ -甲基烯酰胺基团。Myers烷基化用于确保两种RCM前体的C2立体化学来自共同的(9 S)-C3-C9烷基碘。通过旋光度数据的比较,Laingolide B的绝对构型已指定为(2 S,9 R)。


























 京公网安备 11010802027423号
京公网安备 11010802027423号