当前位置:
X-MOL 学术
›
RSC Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Assessment of the trifluoromethyl ketone functionality as an alternative zinc-binding group for selective HDAC6 inhibition†
RSC Medicinal Chemistry ( IF 4.1 ) Pub Date : 2018-05-18 00:00:00 , DOI: 10.1039/c8md00107c Yves Depetter 1, 2, 3 , Silke Geurs 1 , Flore Vanden Bussche 1 , Rob De Vreese 1 , Jorick Franceus 4 , Tom Desmet 4 , Olivier De Wever 2, 3 , Matthias D'hooghe 1
RSC Medicinal Chemistry ( IF 4.1 ) Pub Date : 2018-05-18 00:00:00 , DOI: 10.1039/c8md00107c Yves Depetter 1, 2, 3 , Silke Geurs 1 , Flore Vanden Bussche 1 , Rob De Vreese 1 , Jorick Franceus 4 , Tom Desmet 4 , Olivier De Wever 2, 3 , Matthias D'hooghe 1
Affiliation
|
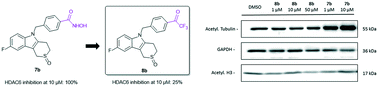
Recent studies point towards the possible disadvantages of using hydroxamic acid-based zinc-binding groups in HDAC inhibitors due to e.g. mutagenicity issues. In this work, we elaborated on our previously developed Tubathian series, a class of highly selective thiaheterocyclic HDAC6 inhibitors, by replacing the benzohydroxamic acid function by an alternative zinc chelator, i.e., an aromatic trifluoromethyl ketone. Unfortunately, these compounds showed a reduced potency to inhibit HDAC6 as compared to their hydroxamic acid counterparts. In agreement, the most active trifluoromethyl ketone was unable to influence the growth of SK-OV-3 ovarian cancer cells nor to alter the acetylation status of tubulin and histone H3. These data suggest that replacement of the zinc-binding hydroxamic acid function with a trifluoromethyl ketone zinc-binding moiety within reported benzohydroxamic HDAC6 inhibitors should not be considered as a standard strategy in HDAC inhibitor development.
中文翻译:

评估三氟甲基酮作为替代锌结合基团选择性抑制 HDAC6 的功能†
最近的研究指出,由于致突变性等问题,在 HDAC 抑制剂中使用基于异羟肟酸的锌结合基团可能存在缺点。在这项工作中,我们通过用另一种锌螯合剂(即芳香族三氟甲基酮)取代苯并异羟肟酸功能,详细阐述了我们之前开发的Tubathian系列,一类高选择性硫杂环HDAC6抑制剂。不幸的是,与异羟肟酸对应物相比,这些化合物抑制 HDAC6 的效力较低。一致地,最活跃的三氟甲基酮既不能影响 SK-OV-3 卵巢癌细胞的生长,也不能改变微管蛋白和组蛋白 H3 的乙酰化状态。这些数据表明,在已报道的苯并异羟肟酸 HDAC6 抑制剂中,用三氟甲基酮锌结合部分取代锌结合异羟肟酸功能不应被视为 HDAC 抑制剂开发中的标准策略。
更新日期:2018-05-18
中文翻译:

评估三氟甲基酮作为替代锌结合基团选择性抑制 HDAC6 的功能†
最近的研究指出,由于致突变性等问题,在 HDAC 抑制剂中使用基于异羟肟酸的锌结合基团可能存在缺点。在这项工作中,我们通过用另一种锌螯合剂(即芳香族三氟甲基酮)取代苯并异羟肟酸功能,详细阐述了我们之前开发的Tubathian系列,一类高选择性硫杂环HDAC6抑制剂。不幸的是,与异羟肟酸对应物相比,这些化合物抑制 HDAC6 的效力较低。一致地,最活跃的三氟甲基酮既不能影响 SK-OV-3 卵巢癌细胞的生长,也不能改变微管蛋白和组蛋白 H3 的乙酰化状态。这些数据表明,在已报道的苯并异羟肟酸 HDAC6 抑制剂中,用三氟甲基酮锌结合部分取代锌结合异羟肟酸功能不应被视为 HDAC 抑制剂开发中的标准策略。



























 京公网安备 11010802027423号
京公网安备 11010802027423号