当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Mechanistic Study of the Reactions of Methyl Peroxy Radical with Methanol or Hydroxyl Methyl Radical
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2018-05-17 00:00:00 , DOI: 10.1021/acs.jpca.7b09988 Zhongrui Zhao 1 , Jinou Song 1 , Boyang Su 1 , Xiaowen Wang 1 , Zhijun Li 1
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2018-05-17 00:00:00 , DOI: 10.1021/acs.jpca.7b09988 Zhongrui Zhao 1 , Jinou Song 1 , Boyang Su 1 , Xiaowen Wang 1 , Zhijun Li 1
Affiliation
|
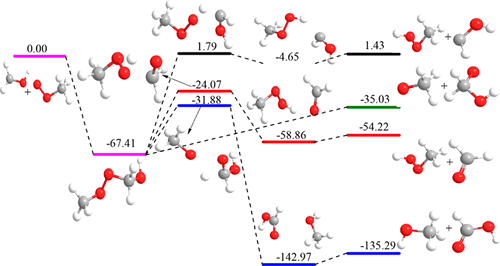
An ab initio and direct dynamic study of the reactions of CH3O2 + CH3OH and CH3O2 + CH2OH has been carried out over the temperature range of 300–1500 K. All stationary points were calculated at the MP2/aug-cc-pVTZ level of theory for CH3O2 + CH3OH or at the M06-2X/MG3S level of theory for CH3O2 + CH2OH and identified for the local minimum. The energetic parameters were refined at the QCISD(T)/cc-pVTZ and CCSD(T)/aug-cc-pVTZ levels of theory. For the reaction of CH3OO + CH3OH, two hydrogen abstraction channels producing CH3OOH + CH2OH (R1) and CH3OOH + CH3O (R2) were confirmed. These two channels consist of the same reversible first step involving the formation of a prereactive complex in the entrance channel. The rate constants of these two channels have been calculated by canonical transition station theory (TST) and canonical variational transition station theory (VTST) with Eckart tunneling correction and compared with the available literature data. The positive temperature dependence of the rate constants was observed. The tunneling effect is important at low temperature and decreases with an increase of the temperature. The contribution of R1 to the total rate constant is dominant, with branching ratios of 0.93 at 500 K and 0.67 at 1000 K, although the branching ratio for R2 increases dramatically with the increase of the temperature from 500 K. For the reaction of CH3OO + CH2OH, eight channels were explored on the lowest singlet and triplet surfaces, and an excited intermediate was found to be formed on the singlet surface. A channel proceeding through the formation of an excited intermediate followed by its impulsive dissociation was confirmed as the dominant channels with a branching ratio more than 0.99 in the temperature range of 300–1500 K, where products of CH3O and OCH2OH were given. The rate constant of the dominant channel calculated by multichannel RRKM-VTST is comparable with the available literature data.
中文翻译:

甲基过氧自由基与甲醇或羟甲基自由基反应的机理研究
在300–1500 K的温度范围内,对CH 3 O 2 + CH 3 OH和CH 3 O 2 + CH 2 OH的反应进行了从头开始和直接动力学的研究。所有固定点均在MP2上计算得出CH 3 O 2 + CH 3 OH的理论水平为/ aug-cc-pVTZ或CH 3 O 2 + CH 2 OH的理论水平为M06-2X / MG3S,并确定为局部最小值。在QCISD(T)/ cc-pVTZ和CCSD(T)/ aug-cc-pVTZ的理论水平上完善了能量参数。用于CH 3 OO + CH 3的反应OH,两个产生氢的通道,分别生成CH 3 OOH + CH 2 OH(R1)和CH 3 OOH + CH 3确认为O(R2)。这两个通道由相同的可逆第一步组成,该步骤涉及在入口通道中形成预反应复合物。这两个通道的速率常数已通过具有Eckart隧道校正的规范过渡站理论(TST)和规范变分过渡站理论(VTST)进行了计算,并与现有文献数据进行了比较。观察到速率常数的正温度依赖性。隧道效应在低温下很重要,并且随着温度的升高而降低。R1对总速率常数的贡献占主导地位,在500 K时支化比为0.93,在1000 K时为0.67,尽管随着温度从500 K升高,R2的支化比急剧增加。3 OO + CH 2 OH,在最低的单重态和三重态表面上探索了八个通道,发现在单重态表面上形成了受激发的中间体。在300–1500 K的温度范围内,通过形成激发的中间体然后进行脉冲解离的通道被证实是支化比大于0.99的主要通道,其中给出了CH 3 O和OCH 2 OH的产物。。通过多通道RRKM-VTST计算出的主导通道的速率常数与现有文献数据相当。
更新日期:2018-05-17
中文翻译:

甲基过氧自由基与甲醇或羟甲基自由基反应的机理研究
在300–1500 K的温度范围内,对CH 3 O 2 + CH 3 OH和CH 3 O 2 + CH 2 OH的反应进行了从头开始和直接动力学的研究。所有固定点均在MP2上计算得出CH 3 O 2 + CH 3 OH的理论水平为/ aug-cc-pVTZ或CH 3 O 2 + CH 2 OH的理论水平为M06-2X / MG3S,并确定为局部最小值。在QCISD(T)/ cc-pVTZ和CCSD(T)/ aug-cc-pVTZ的理论水平上完善了能量参数。用于CH 3 OO + CH 3的反应OH,两个产生氢的通道,分别生成CH 3 OOH + CH 2 OH(R1)和CH 3 OOH + CH 3确认为O(R2)。这两个通道由相同的可逆第一步组成,该步骤涉及在入口通道中形成预反应复合物。这两个通道的速率常数已通过具有Eckart隧道校正的规范过渡站理论(TST)和规范变分过渡站理论(VTST)进行了计算,并与现有文献数据进行了比较。观察到速率常数的正温度依赖性。隧道效应在低温下很重要,并且随着温度的升高而降低。R1对总速率常数的贡献占主导地位,在500 K时支化比为0.93,在1000 K时为0.67,尽管随着温度从500 K升高,R2的支化比急剧增加。3 OO + CH 2 OH,在最低的单重态和三重态表面上探索了八个通道,发现在单重态表面上形成了受激发的中间体。在300–1500 K的温度范围内,通过形成激发的中间体然后进行脉冲解离的通道被证实是支化比大于0.99的主要通道,其中给出了CH 3 O和OCH 2 OH的产物。。通过多通道RRKM-VTST计算出的主导通道的速率常数与现有文献数据相当。



























 京公网安备 11010802027423号
京公网安备 11010802027423号