European Journal of Medicinal Chemistry ( IF 6.7 ) Pub Date : 2018-05-18 , DOI: 10.1016/j.ejmech.2018.05.022 William Nguyen , Anthony N. Hodder , Richard Bestel de Lezongard , Peter E. Czabotar , Kate E. Jarman , Matthew T. O'Neill , Jennifer K. Thompson , Helene Jousset Sabroux , Alan F. Cowman , Justin A. Boddey , Brad E. Sleebs
|
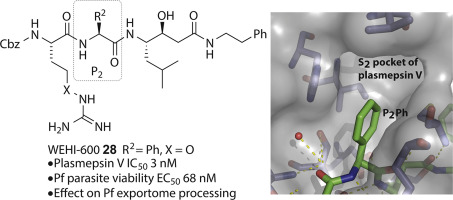
Plasmepsin V is an aspartyl protease that plays a critical role in the export of proteins bearing the Plasmodium export element (PEXEL) motif (RxLxQ/E/D) to the infected host erythrocyte, and thus the survival of the malaria parasite. Previously, development of transition state PEXEL mimetic inhibitors of plasmepsin V have primarily focused on demonstrating the importance of the P3 Arg and P1 Leu in binding affinity and selectivity. Here, we investigate the importance of the P2 position by incorporating both natural and non-natural amino acids into this position and show disubstituted beta-carbon amino acids convey the greatest potency. Consequently, we show analogues with either cyclohexylglycine or phenylglycine in the P2 position are the most potent inhibitors of plasmepsin V that impair processing of the PEXEL motif in exported proteins resulting in death of P. falciparum asexual stage parasites.
中文翻译:

通过修改PEXEL拟肽的P 2位置来增强纤溶酶V抑制剂的抗疟活性
纤溶酶V是一种天冬氨酰蛋白酶,在携带带有疟原虫输出元件(PEXEL)基序的蛋白质(RxLxQ / E / D)出口到受感染的宿主红细胞以及疟疾寄生虫的存活中起关键作用。以前,纤溶酶V的过渡态PEXEL模拟抑制剂的开发主要集中于证明P 3 Arg和P 1 Leu在结合亲和力和选择性方面的重要性。在这里,我们通过将天然和非天然氨基酸都掺入该位置来研究P 2位置的重要性,并表明双取代的β-碳氨基酸可发挥最大的效能。因此,我们在P中显示了带有环己基甘氨酸或苯基甘氨酸的类似物2位是最有效的纤溶酶V抑制剂,可破坏输出蛋白中PEXEL基序的加工,导致恶性疟原虫无性阶段寄生虫死亡。



























 京公网安备 11010802027423号
京公网安备 11010802027423号