当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
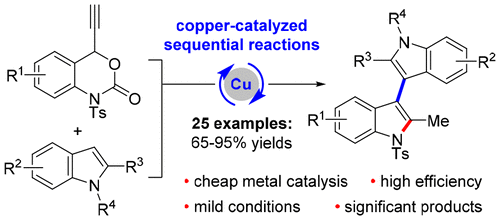
Synthesis of 3,3′-Biindoles through a Copper-Catalyzed Friedel–Crafts Propargylation/Hydroamination/Aromatization Sequence
Organic Letters ( IF 5.2 ) Pub Date : 2018-05-17 00:00:00 , DOI: 10.1021/acs.orglett.8b01100 Tian-Ren Li 1 , Mao-Mao Zhang 1 , Bao-Cheng Wang 1 , Liang-Qiu Lu 1 , Wen-Jing Xiao 1, 2
Organic Letters ( IF 5.2 ) Pub Date : 2018-05-17 00:00:00 , DOI: 10.1021/acs.orglett.8b01100 Tian-Ren Li 1 , Mao-Mao Zhang 1 , Bao-Cheng Wang 1 , Liang-Qiu Lu 1 , Wen-Jing Xiao 1, 2
Affiliation
|
A copper-catalyzed Friedel–Crafts propargylation/hydroamination/aromatization sequence is described. In the presence of a catalytic amount of CuI, this sequential reaction can convert ethynyl benzoxazinanones and indoles into a diverse set of 3,3′-biindoles with high efficiency and selectivity. Moreover, the synthesis of other indole–heteroaryl molecules and the catalytic asymmetric formation of axially chiral 3,3′-biindoles are demonstrated.
中文翻译:

通过铜催化的Friedel-Crafts炔丙基化/氢化/芳香化序列合成3,3'-联吲哚
描述了铜催化的Friedel-Crafts炔丙基化/加氢胺化/芳构化顺序。在催化量的CuI的存在下,该顺序反应可以高效高效地将乙炔基苯并恶嗪酮和吲哚转化为各种3,3'-联吲哚。此外,还证明了其他吲哚-杂芳基分子的合成和轴向手性3,3'-双吲哚的催化不对称形成。
更新日期:2018-05-17
中文翻译:

通过铜催化的Friedel-Crafts炔丙基化/氢化/芳香化序列合成3,3'-联吲哚
描述了铜催化的Friedel-Crafts炔丙基化/加氢胺化/芳构化顺序。在催化量的CuI的存在下,该顺序反应可以高效高效地将乙炔基苯并恶嗪酮和吲哚转化为各种3,3'-联吲哚。此外,还证明了其他吲哚-杂芳基分子的合成和轴向手性3,3'-双吲哚的催化不对称形成。



























 京公网安备 11010802027423号
京公网安备 11010802027423号