Chem ( IF 23.5 ) Pub Date : 2018-05-17 , DOI: 10.1016/j.chempr.2018.04.012 Yu Tang , Jian Xu , Jian Yang , Lili Lin , Xiaoming Feng , Xiaohua Liu
|
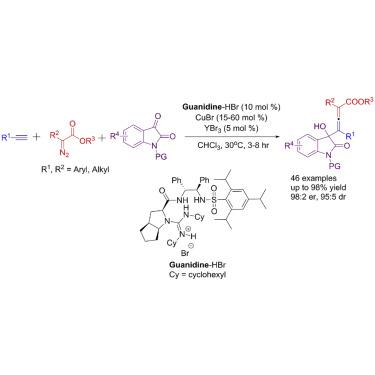
A catalytic asymmetric three-component reaction of α-diazoesters with terminal alkynes and isatins was achieved. This one-pot synthesis gave rise to axially chiral tetrasubstituted allenoates bearing a stereogenic center. The chiral guanidinium salt/CuBr/YBr3 catalytic system proved efficient and highly diastereo- and enantioselective for a wide range of alkynes, aromatic α-diazoesters, and isatins under mild reaction conditions. This approach enables a Cu(I)-involved asymmetric multicomponent reaction (AMCR) of α-diazo compounds and gives solid experimental evidence for the formation of allenoate-Cu(I) intermediates in C–H insertion of α-diazoesters to terminal alkynes. We also found that additional acids improved the catalyst efficiency of guanidinium salt/CuCl in the direct enantioselective C–H insertion of α-aryl diazoesters. Mechanism studies suggest that the combined-acid systems (Lewis acid combined with assisted Lewis acid or Brønsted acid combined with assisted Lewis acid) bring out higher reactivity by associative interaction and allow for an equally effective asymmetric environment.
中文翻译:

非对称三组分反应通过脲酸酯-铜中间体合成四取代的脲酸酯
实现了α-重氮酸酯与末端炔烃和靛红的催化不对称三组分反应。这种一锅合成法产生了带有手性中心的轴向手性四取代的脲酸酯。手性胍盐/ CuBr / YBr 3在温和的反应条件下,催化体系被证明对多种炔烃,芳族α-重氮酯和靛红具有高非对映选择性和对映选择性。这种方法可以使α-重氮化合物发生Cu(I)参与的不对称多组分反应(AMCR),并为α-重氮酯在末端炔烃的C–H插入中形成烯丙酸酯-Cu(I)中间体提供了可靠的实验证据。我们还发现,在α-芳基重氮酯的直接对映选择性C–H插入中,其他酸可提高胍盐/ CuCl的催化效率。机理研究表明,组合酸体系(路易斯酸与辅助路易斯酸结合或布朗斯台德酸与辅助路易斯酸结合)通过缔合相互作用带来更高的反应性,并允许同样有效的不对称环境。


























 京公网安备 11010802027423号
京公网安备 11010802027423号