Acta Biomaterialia ( IF 9.7 ) Pub Date : 2018-05-10 , DOI: 10.1016/j.actbio.2018.05.015 Yonghui Ding , Xin Xu , Sadhana Sharma , Michael Floren , Kurt Stenmark , Stephanie J. Bryant , Corey P. Neu , Wei Tan
|
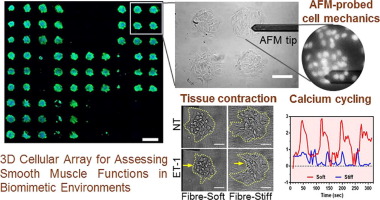
The ability to assess changes in smooth muscle contractility and pharmacological responsiveness in normal or pathological-relevant vascular tissue environments is critical to enable vascular drug discovery. However, major challenges remain in both capturing the complexity of in vivo vascular remodeling and evaluating cell contractility in complex, tissue-like environments. Herein, we developed a biomimetic fibrous hydrogel with tunable structure, stiffness, and composition to resemble the native vascular tissue environment. This hydrogel platform was further combined with the combinatory protein array technology as well as advanced approaches to measure cell mechanics and contractility, thus permitting evaluation of smooth muscle functions in a variety of tissue-like microenvironments. Our results demonstrated that biomimetic fibrous structure played a dominant role in smooth muscle function, while the presentation of adhesion proteins co-regulated it to various degrees. Specifically, fibre networks enabled cell infiltration and upregulated expression of actomyosin proteins in contrast to flat hydrogels. Remarkably, fibrous structure and physiologically relevant stiffness of hydrogels cooperatively enhanced smooth muscle contractility and pharmacological responses to vasoactive drugs at both the single cell and intact tissue levels. Together, this study is the first to demonstrate alterations of human vascular smooth muscle contractility and pharmacological responsiveness in biomimetic soft, fibrous environments with a cellular array platform. The integrated platform produced here could enable investigations for pathobiology and pharmacological interventions by developing a broad range of patho-physiologically relevant in vitro tissue models.
Statement of Significance
Engineering functional smooth muscle in vitro holds the great potential for diseased tissue replacement and drug testing. A central challenge is recapitulating the smooth muscle contractility and pharmacological responses given its significant phenotypic plasticity in response to changes in environment. We present a biomimetic fibrous hydrogel with tunable structure, stiffness, and composition that enables the creation of functional smooth muscle tissues in the native vascular tissue-like microenvironment. Such fibrous hydrogel is further combined with the combinatory protein array technology to construct cellular array for evaluation of smooth muscle phenotype, contraction, and cell mechanics. The integrated platform produced here could be promising for developing a broad range of normal or diseased in vitro tissue models.
中文翻译:

仿生软纤维水凝胶,用于收缩和药理反应性平滑肌
在正常或病理相关的血管组织环境中评估平滑肌收缩力和药理反应性变化的能力对于实现血管药物发现至关重要。然而,在捕获体内复杂性方面仍然存在重大挑战在复杂的组织状环境中进行血管重塑和评估细胞收缩力。在这里,我们开发了一种仿生纤维水凝胶,其结构,刚度和组成均可调,类似于天然的血管组织环境。该水凝胶平台进一步与组合蛋白阵列技术以及用于测量细胞力学和收缩力的先进方法相结合,从而可以评估各种组织样微环境中的平滑肌功能。我们的结果表明,仿生纤维结构在平滑肌功能中起主要作用,而粘附蛋白的表达则在不同程度上共同调节了它。具体而言,与扁平水凝胶相反,纤维网络能够使细胞浸润,并且放线肌球蛋白的表达上调。值得注意的是 水凝胶的纤维结构和生理相关的硬度在单个细胞水平和完整组织水平上共同增强了平滑肌的收缩性和对血管活性药物的药理反应。总之,这项研究首次证明了在具有细胞阵列平台的仿生柔软纤维环境中人类血管平滑肌收缩力和药理反应性的改变。这里开发的集成平台可以通过开发广泛的病理生理相关因素,来进行病理生物学和药理学干预研究 这项研究首次证明了在具有细胞阵列平台的仿生柔软纤维环境中,人类血管平滑肌收缩力和药理反应性的改变。这里开发的集成平台可以通过开发广泛的病理生理相关因素,来进行病理生物学和药理学干预研究 这项研究首次证明了在具有细胞阵列平台的仿生柔软纤维环境中,人类血管平滑肌收缩力和药理反应性的改变。这里开发的集成平台可以通过开发广泛的病理生理相关因素,来进行病理生物学和药理学干预研究体外组织模型。
重要声明
在体外工程化功能性平滑肌在患病组织更换和药物测试方面具有巨大潜力。鉴于其对环境变化的显着表型可塑性,一个主要的挑战是概括平滑肌的收缩力和药理反应。我们提出了具有可调结构,刚度和组成的仿生纤维水凝胶,该凝胶能够在天然血管组织样微环境中创建功能性平滑肌组织。将这种纤维水凝胶进一步与组合蛋白阵列技术相结合,以构建用于评估平滑肌表型,收缩和细胞力学的细胞阵列。这里生产的集成平台可能会为开发各种正常或患病的人带来希望体外组织模型。



























 京公网安备 11010802027423号
京公网安备 11010802027423号