当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Redox-neutral tri-/difluoromethylation of para-quinone methides with sodium sulfinates†
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2018-05-17 00:00:00 , DOI: 10.1039/c8qo00428e Qing-Yan Wu 1, 2, 3, 4, 5 , Gui-Zhen Ao 1, 2, 3, 4, 5 , Feng Liu 1, 2, 3, 4, 5
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2018-05-17 00:00:00 , DOI: 10.1039/c8qo00428e Qing-Yan Wu 1, 2, 3, 4, 5 , Gui-Zhen Ao 1, 2, 3, 4, 5 , Feng Liu 1, 2, 3, 4, 5
Affiliation
|
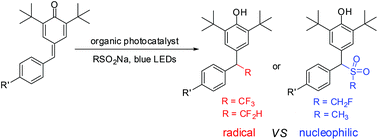
The radical tri-/difluoromethylation of para-quinone methides with readily available sodium tri-/difluoromethanesulfinate via organic photoredox catalysis is described. This reaction is external oxidant free and exhibits wide functional group compatibility, providing the desired products in useful yields. However, the reaction of para-quinone methides with CH2FSO2Na and CH3SO2Na under the optimal conditions gives the nucleophilic conjugate addition products. The results indicate that the strong inductive effect of the fluorine atom may play a critical role in the reactivity of the sodium sulfinates.
中文翻译:

对亚醌甲基化物与亚磺酸钠的 氧化还原中性三/二氟甲基化†
描述了通过有机光氧化还原催化用容易获得的三-/二氟甲磺酸钠与对-醌甲基的自由基三-/二氟甲基化。该反应不含外部氧化剂,并具有广泛的官能团相容性,以有用的收率提供了所需的产物。但是,在最佳条件下,对苯醌甲基化物与CH 2 FSO 2 Na和CH 3 SO 2 Na的反应产生了亲核偶联物加成产物。结果表明,氟原子的强诱导作用可能在亚磺酸钠的反应性中起关键作用。
更新日期:2018-05-17
中文翻译:

对亚醌甲基化物与亚磺酸钠的 氧化还原中性三/二氟甲基化†
描述了通过有机光氧化还原催化用容易获得的三-/二氟甲磺酸钠与对-醌甲基的自由基三-/二氟甲基化。该反应不含外部氧化剂,并具有广泛的官能团相容性,以有用的收率提供了所需的产物。但是,在最佳条件下,对苯醌甲基化物与CH 2 FSO 2 Na和CH 3 SO 2 Na的反应产生了亲核偶联物加成产物。结果表明,氟原子的强诱导作用可能在亚磺酸钠的反应性中起关键作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号