Bioorganic Chemistry ( IF 5.1 ) Pub Date : 2018-05-02 , DOI: 10.1016/j.bioorg.2018.04.029 Ayaz Mahmood Dar , Rizwan Nabi , Shafia Mir , Manzoor Ahmad Gatoo , Shamsuzzaman , Shabir H. Lone
|
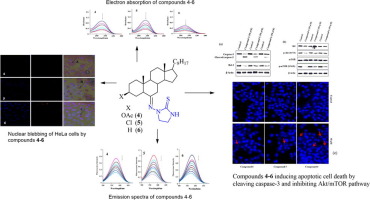
New steroidal imidazolidinthione derivatives (4–6) were synthesized from steroidal thiosemicarbazones and dichloroethane. The synthesized compounds were characterized using spectral data analysis. Theoretical DFT involving B3LYP/6-31G∗∗ level of theory was employed to gain insights into the molecular structure of the target compounds. MEPS and FMO analysis were carried out. HOMO-LUMO energy gap was determined which helped to evaluate various global descriptors like hardness, chemical potential, electronegativity, nucleophilicity and electrophilicity index, etc. The calculated properties established that the synthesized products are more or less similar in their reactivity behaviour. To explore their biological potential, interaction studies of compounds (4–6) with DNA were carried out using various biophysical techniques. The compounds bind DNA preferentially through electrostatic and hydrophobic interactions with Kb of 3.21 × 103 M−1, 2.79 × 103 M−1 and 2.26 × 103 M−1, respectively indicating the higher binding affinity of compound 4 towards DNA. Gel electrophoresis of compound 4 demonstrated strong interaction during the concentration dependent cleavage activity with pBR322 DNA. It was observed that these steroidal imidazolidinthiones are minor groove binders of DNA which was validated using molecular docking studies. An in vitro cytotoxicity screening using MTT assay revealed that the compounds (4–6) exhibit potential toxicity against different human cancer cells. Highest antiproliferative effect was observed on HeLa cells by compound 4. The results suggested that compounds 4–6 cause apoptotic cell death by cleaving apoptotic protein caspase-3 and suppress anti-apoptotic protein Bcl-2 in HeLa cancer cells.
中文翻译:

甾族咪唑烷硫酮类化合物作为潜在的凋亡因子的合成:通过理论和实验研究
新甾族imidazolidinthione衍生物(4 - 6)从缩氨基硫脲类固醇和二氯乙烷合成。使用光谱数据分析对合成的化合物进行表征。涉及理论水平B3LYP / 6-31G ***的理论DFT被用来深入了解目标化合物的分子结构。进行了MEPS和FMO分析。确定了HOMO-LUMO的能隙,这有助于评估各种全局指标,如硬度,化学势,电负性,亲核性和亲电性指数等。计算的性质确定了合成产物的反应性行为或多或少相似。为了探索它们的生物学潜能,化合物之间的相互作用研究(4 –6)使用各种生物物理技术进行DNA。优先通过使用K静电和疏水性相互作用的化合物结合DNA b的3.21×10 3中号-1,2.79×10 3中号-1和2.26×10 3中号-1,分别表示化合物的较高结合亲和力4朝DNA。化合物4的凝胶电泳显示在与pBR322 DNA的浓度依赖性切割活性过程中有很强的相互作用。观察到这些甾族咪唑烷硫酮是DNA的小沟结合物,其已通过分子对接研究得到证实。一种在体外细胞毒性用MTT法筛选,发现该化合物(4 - 6)表现出针对不同人癌细胞的潜在毒性。化合物4对HeLa细胞具有最高的抗增殖作用。结果表明,化合物4 – 6通过裂解凋亡蛋白caspase-3并抑制HeLa癌细胞中的抗凋亡蛋白Bcl-2导致凋亡性细胞死亡。


























 京公网安备 11010802027423号
京公网安备 11010802027423号