当前位置:
X-MOL 学术
›
Chem. Bio. Drug Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis and antibacterial activities of novel pleuromutilin derivatives bearing an aminothiophenol moiety
Chemical Biology & Drug Design ( IF 3 ) Pub Date : 2018-05-31 , DOI: 10.1111/cbdd.13328 Zhao-Sheng Zhang 1 , Yun-Zhen Huang 1 , Jian Luo 1 , Zhen Jin 1 , Ya-Hong Liu 1 , You-Zhi Tang 1
Chemical Biology & Drug Design ( IF 3 ) Pub Date : 2018-05-31 , DOI: 10.1111/cbdd.13328 Zhao-Sheng Zhang 1 , Yun-Zhen Huang 1 , Jian Luo 1 , Zhen Jin 1 , Ya-Hong Liu 1 , You-Zhi Tang 1
Affiliation
|
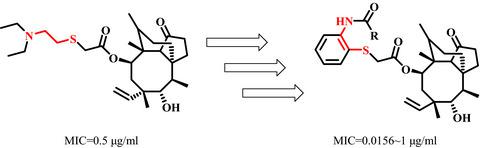
We synthesized a series of novel thioether pleuromutilin derivatives incorporating 2‐aminothiophenol moieties into the C14 side chain via acylation reactions under mild conditions. We evaluated the in‐vitro antibacterial activities of the derivatives against methicillin‐resistant Staphylococcus aureus (MRSA, ATCC 43300), Staphylococcus aureus (ATCC 29213) and Escherichia coli (ATCC 25922). The majority of the synthesized derivatives possessed moderate antibacterial activities. Compound 8 was found to be the most active antibacterial derivative against MRSA. We conducted docking experiments to understand the possible mode of interactions between compounds 8, 9b, 11a and 50S ribosomal subunit. The docking results proved that there is a reasonable correlation between the binding free energy and the antibacterial activity. Compound 8 was evaluated for its in‐vivo antibacterial activity and showed higher efficacy than tiamulin against MRSA in mouse infection model.
中文翻译:

具有氨基硫酚部分的新型截短侧耳素衍生物的合成和抗菌活性
我们合成了一系列新颖的硫醚截短侧耳素衍生物,它们在温和的条件下通过酰化反应将2-氨基硫酚部分掺入C14侧链。我们评估了该衍生物对耐甲氧西林金黄色葡萄球菌(MRSA,ATCC 43300),金黄色葡萄球菌(ATCC 29213)和大肠杆菌(ATCC 25922)的体外抗菌活性。大多数合成的衍生物具有中等的抗菌活性。发现化合物8是针对MRSA的最具活性的抗菌衍生物。我们进行对接实验理解的相互作用的化合物之间的可能的模式8,图9B,图11A和50S核糖体亚基。对接结果证明,结合自由能与抗菌活性之间存在合理的相关性。对化合物8的体内抗菌活性进行了评估,并在小鼠感染模型中显示出比头孢菌素更高的抗MRSA功效。
更新日期:2018-05-31
中文翻译:

具有氨基硫酚部分的新型截短侧耳素衍生物的合成和抗菌活性
我们合成了一系列新颖的硫醚截短侧耳素衍生物,它们在温和的条件下通过酰化反应将2-氨基硫酚部分掺入C14侧链。我们评估了该衍生物对耐甲氧西林金黄色葡萄球菌(MRSA,ATCC 43300),金黄色葡萄球菌(ATCC 29213)和大肠杆菌(ATCC 25922)的体外抗菌活性。大多数合成的衍生物具有中等的抗菌活性。发现化合物8是针对MRSA的最具活性的抗菌衍生物。我们进行对接实验理解的相互作用的化合物之间的可能的模式8,图9B,图11A和50S核糖体亚基。对接结果证明,结合自由能与抗菌活性之间存在合理的相关性。对化合物8的体内抗菌活性进行了评估,并在小鼠感染模型中显示出比头孢菌素更高的抗MRSA功效。


























 京公网安备 11010802027423号
京公网安备 11010802027423号