当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Total Synthesis of the Marine Macrolide Amphidinolide F
Organic Letters ( IF 5.2 ) Pub Date : 2018-05-15 00:00:00 , DOI: 10.1021/acs.orglett.8b01020 Laurent Ferrié 1 , Johan Fenneteau 1 , Bruno Figadère 1
Organic Letters ( IF 5.2 ) Pub Date : 2018-05-15 00:00:00 , DOI: 10.1021/acs.orglett.8b01020 Laurent Ferrié 1 , Johan Fenneteau 1 , Bruno Figadère 1
Affiliation
|
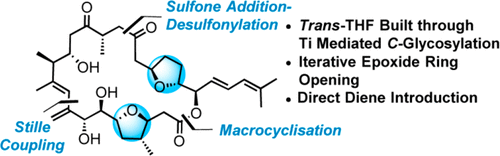
A new and efficient convergent approach toward the synthesis of amphidinolide F is described through the assembly of three fragments. The two trans-tetrahydrofurans were built by a diastereoselective C-glycosylation with titanium enolate of bulky N-acetyloxazolidinethiones. The side chain was inserted by a Liebeskind–Srogl cross-coupling reaction. A sulfone condensation/desulfonylation sequence, a Stille cross-coupling, and a macrolactonization were applied to connect the fragments.
中文翻译:

海洋大环内酯Amphidinolide F的全合成
通过组装三个片段描述了一种新的有效的融合方法,用于合成两性霉素F。两种反式-四氢呋喃是通过与大体积N-乙酰氧基恶唑烷硫酮的烯醇钛的非对映选择性C-糖基化而构建的。侧链通过Liebeskind-Srogl交叉偶联反应插入。使用砜缩合/脱磺酰基顺序,Stille交叉偶联和大内酯化作用来连接片段。
更新日期:2018-05-15
中文翻译:

海洋大环内酯Amphidinolide F的全合成
通过组装三个片段描述了一种新的有效的融合方法,用于合成两性霉素F。两种反式-四氢呋喃是通过与大体积N-乙酰氧基恶唑烷硫酮的烯醇钛的非对映选择性C-糖基化而构建的。侧链通过Liebeskind-Srogl交叉偶联反应插入。使用砜缩合/脱磺酰基顺序,Stille交叉偶联和大内酯化作用来连接片段。



























 京公网安备 11010802027423号
京公网安备 11010802027423号