当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Polyoxometalate-Supported Bis(2,2′-bipyridine)mono(aqua)nickel(II) Coordination Complex: an Efficient Electrocatalyst for Water Oxidation
Inorganic Chemistry ( IF 4.6 ) Pub Date : 2018-05-15 00:00:00 , DOI: 10.1021/acs.inorgchem.8b00541 Chandani Singh 1 , Subhabrata Mukhopadhyay 1 , Samar K. Das 1
Inorganic Chemistry ( IF 4.6 ) Pub Date : 2018-05-15 00:00:00 , DOI: 10.1021/acs.inorgchem.8b00541 Chandani Singh 1 , Subhabrata Mukhopadhyay 1 , Samar K. Das 1
Affiliation
|
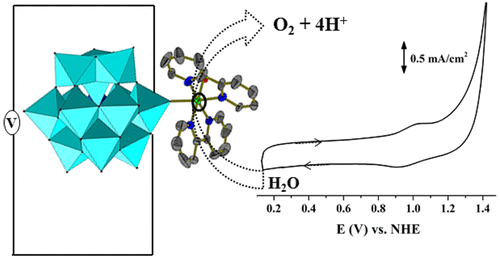
A polyoxometalate (POM)-supported nickel(II) coordination complex, [NiII(2,2′-bpy)3]3[{NiII(2,2′-bpy)2(H2O)}{HCoIIWVI12O40}]2·3H2O (1; 2,2′-bpy = 2,2′-bipyridine), has been synthesized and structurally characterized. We could obtain a relatively better resolved structure from dried crystals of 1, NiII(2,2′-bpy)3]3[{NiII(2,2′-bpy)2(H2O)}{HCoIIWVI12O40}]2·H2O (D1). Because the title compound has been characterized with a {NiII(2,2′-bpy)2(H2O)}2+ fragment coordinated to the surface of the Keggin anion ([H(CoIIW12O40]5–) via a terminal oxo group of tungsten and the [NiII(2,2′-bpy)3]2+ coordination complex cation sitting as the lattice component in the concerned crystals, the electronic spectroscopy of compound 1 has been described by comparing its electronic spectral features with those of [NiII(2,2′-bpy)2(H2O)Cl]Cl, [NiII(2,2′-bpy)3]Cl2, and K6[CoIIW12O40]·6H2O. Most importantly, compound 1 can function as a heterogeneous and robust electrochemical water oxidation catalyst (WOC). To gain insights into the water oxidation (WO) protocol and to interpret the nature of the active catalyst, diverse electrochemical experiments have been conducted. The mode of action of the WOC during the electrochemical process is accounted for by confirmation that there was no formation/participation of metal oxide during various controlled experiments. It is found that the title compound acts as a true catalyst that has NiII (coordinated to POM surface) acting as the active catalytic center. It is also found to follow a proton-coupled electron-transfer pathway (two electrons and one proton) for WO catalysis with a high turnover frequency of 18.49 (mol of O2)(mol of NiII)−1 s–1.
中文翻译:

多金属氧酸盐负载的双(2,2'-联吡啶)单(水)镍(II)配合物:水氧化的高效电催化剂
一种多金属氧酸盐(POM)负载的镍(II)配位络合物[Ni II(2,2'-bpy)3 ] 3 [{Ni II(2,2'-bpy)2(H 2 O)} {HCo II W VI 12 O 40 }] 2 ·3H 2 O(1 ; 2,2'-bpy = 2,2'-联吡啶)已合成并进行了结构表征。我们可以从1,Ni II(2,2'-bpy)3 ] 3 [{Ni II(2,2'-bpy)2(H 2O)} {HCo II W VI 12 O 40 }] 2 ·H 2 O(D1)。因为标题化合物的特征是与Keggin阴离子的表面配位的{Ni II(2,2'-bpy)2(H 2 O)} 2+片段([H(Co II W 12 O 40 ] 5 –)通过钨的末端氧代基和[Ni II(2,2'-bpy)3 ] 2+配位络合物阳离子作为相关晶体中的晶格成分,对该化合物的电子光谱1已经通过与那些比较其电子光谱特征描述的Ni II(2,2'-联吡啶)2(H 2 O)CL]氯,[镍II(2,2'-联吡啶)3 ]氯2,和K 6 [CO II w ^ 12 ö 40 ]·6H 2 O.最重要的是,化合物1可以用作非均相且坚固的电化学水氧化催化剂(WOC)。为了深入了解水氧化(WO)协议并解释活性催化剂的性质,已进行了多种电化学实验。通过确认在各种受控实验期间没有金属氧化物的形成/参与,可以解释WOC在电化学过程中的作用方式。发现标题化合物充当具有Ni II(与POM表面配位)作为活性催化中心的真实催化剂。还发现它遵循质子耦合电子转移途径(两个电子和一个质子)以WO催化,其高周转频率为18.49(mol O 2)(Ni II摩尔)-1 s –1。
更新日期:2018-05-15
中文翻译:

多金属氧酸盐负载的双(2,2'-联吡啶)单(水)镍(II)配合物:水氧化的高效电催化剂
一种多金属氧酸盐(POM)负载的镍(II)配位络合物[Ni II(2,2'-bpy)3 ] 3 [{Ni II(2,2'-bpy)2(H 2 O)} {HCo II W VI 12 O 40 }] 2 ·3H 2 O(1 ; 2,2'-bpy = 2,2'-联吡啶)已合成并进行了结构表征。我们可以从1,Ni II(2,2'-bpy)3 ] 3 [{Ni II(2,2'-bpy)2(H 2O)} {HCo II W VI 12 O 40 }] 2 ·H 2 O(D1)。因为标题化合物的特征是与Keggin阴离子的表面配位的{Ni II(2,2'-bpy)2(H 2 O)} 2+片段([H(Co II W 12 O 40 ] 5 –)通过钨的末端氧代基和[Ni II(2,2'-bpy)3 ] 2+配位络合物阳离子作为相关晶体中的晶格成分,对该化合物的电子光谱1已经通过与那些比较其电子光谱特征描述的Ni II(2,2'-联吡啶)2(H 2 O)CL]氯,[镍II(2,2'-联吡啶)3 ]氯2,和K 6 [CO II w ^ 12 ö 40 ]·6H 2 O.最重要的是,化合物1可以用作非均相且坚固的电化学水氧化催化剂(WOC)。为了深入了解水氧化(WO)协议并解释活性催化剂的性质,已进行了多种电化学实验。通过确认在各种受控实验期间没有金属氧化物的形成/参与,可以解释WOC在电化学过程中的作用方式。发现标题化合物充当具有Ni II(与POM表面配位)作为活性催化中心的真实催化剂。还发现它遵循质子耦合电子转移途径(两个电子和一个质子)以WO催化,其高周转频率为18.49(mol O 2)(Ni II摩尔)-1 s –1。


























 京公网安备 11010802027423号
京公网安备 11010802027423号