当前位置:
X-MOL 学术
›
Chem. Asian J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Enantiospecific Suzuki–Miyaura Coupling of Nonbenzylic α‐(Acylamino)alkylboronic Acid Derivatives
Chemistry - An Asian Journal ( IF 4.1 ) Pub Date : 2018-06-07 , DOI: 10.1002/asia.201800536 Toshimichi Ohmura 1 , Kyoko Miwa 1 , Tomotsugu Awano 1 , Michinori Suginome 1
Chemistry - An Asian Journal ( IF 4.1 ) Pub Date : 2018-06-07 , DOI: 10.1002/asia.201800536 Toshimichi Ohmura 1 , Kyoko Miwa 1 , Tomotsugu Awano 1 , Michinori Suginome 1
Affiliation
|
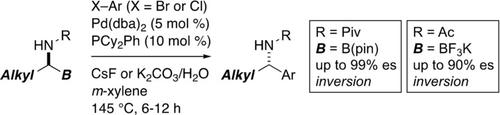
Suzuki–Miyaura coupling of nonbenzylic α‐(acylamino)alkylboron compounds with aryl halides is established. A Pd/PCy2Ph catalyst promotes the reaction efficiently at 145 °C. The reaction of enantioenriched α‐(acylamino)alkylboron compounds affords chiral 1‐arylalkylamides in high enantiospecificity and inversion of configuration.
中文翻译:

非苄基α-(酰基氨基)烷基硼酸衍生物的对映体特异性Suzuki-Miyaura偶联
建立了非苄基α-(酰基氨基)烷基硼化合物与芳基卤化物的Suzuki-Miyaura偶联。Pd / PCy 2 Ph催化剂可在145°C的条件下有效地促进反应。富含对映体的α-(酰基氨基)烷基硼化合物的反应可提供高对映体特异性和构型反转的手性1-芳基烷基酰胺。
更新日期:2018-06-07
中文翻译:

非苄基α-(酰基氨基)烷基硼酸衍生物的对映体特异性Suzuki-Miyaura偶联
建立了非苄基α-(酰基氨基)烷基硼化合物与芳基卤化物的Suzuki-Miyaura偶联。Pd / PCy 2 Ph催化剂可在145°C的条件下有效地促进反应。富含对映体的α-(酰基氨基)烷基硼化合物的反应可提供高对映体特异性和构型反转的手性1-芳基烷基酰胺。


























 京公网安备 11010802027423号
京公网安备 11010802027423号