Water Research ( IF 12.8 ) Pub Date : 2018-05-15 , DOI: 10.1016/j.watres.2018.05.024 Jan Kolařík , Robert Prucek , Jiří Tuček , Jan Filip , Virender K. Sharma , Radek Zbořil
|
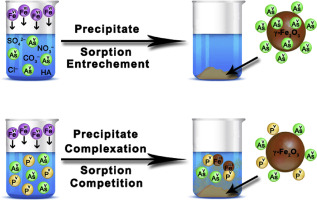
Arsenic compounds are carcinogenic to humans and are typically removed from contaminated water using various sorbents. The ionic composition plays a significant role in arsenate removal efficiency during the process of water remediation. Here, we quantify the effects of natural ions (chlorides, nitrates, carbonates, sulfates, and phosphates) and humic acid on the removal of arsenates by ferrate(VI) at pH = 6.6. In the experiments, the initial concentration of arsenates was 10 mg L−1 (as As) and the concentrations of ions varied in the range from 5 to 100 mg L−1 of element in ion and humic acid. The achieved results show that only phosphates ions had principle influence on the efficiency of arsenate removal by ferrate(VI). The effect of phosphates was elucidated by applying transmission electron microscopy, energy-dispersive X-ray spectroscopy, and low temperature in-field 57Fe Mössbauer spectroscopy to solid samples, prepared under different weight ratios of ferrate(VI), arsenates, and phosphates. These results show three crucial effects of phosphates on the arsenate removal mechanisms. At low P:As weight ratio (up to 1:1), the incorporation of arsenate ions into the crystalline structure of γ-Fe2O3/γ-FeOOH nanoparticles was found to be suppressed by the presence of phosphates. Thus, arsenates were mainly adsorbed onto the surface of γ-Fe2O3/γ-FeOOH nanoparticles. Further increase in the P:As weight ratio (more than 1:1) resulted in the competition between arsenates and phosphates sorption. With the increased concentration of phosphates ions, the number of arsenates on the surface of γ-Fe2O3/γ-FeOOH nanoparticles was reduced. Finally, the complexation of iron(III) ions with phosphates ions occurred, leading to a decrease in the arsenate removal efficiency, which resulted from a lower content of precipitated γ-Fe2O3/γ-FeOOH nanoparticles. All these aspects need to be considered prior to application of ferrate(VI) for arsenate removal in real natural waters.
中文翻译:

无机离子和天然有机物对高铁酸盐去除砷酸盐的影响(VI):了解磷酸根离子的复杂作用
砷化合物对人类具有致癌性,通常使用各种吸附剂将其从污染水中去除。离子组合物在水修复过程中的砷去除效率中起着重要作用。在这里,我们量化了天然离子(氯化物,硝酸盐,碳酸盐,硫酸盐和磷酸盐)和腐殖酸对pH = 6.6时高铁酸盐(VI)去除砷酸盐的影响。在实验中,砷酸盐的初始浓度为10 mg L -1(以As计),离子浓度在5至100 mg L -1的范围内变化。离子和腐殖酸中的元素。所得结果表明,只有磷酸根离子对高铁酸盐(VI)去除砷酸盐的效率有主要影响。通过应用透射电子显微镜,能量色散X射线光谱法和低温现场57 FeMössbauer光谱法对固体样品进行了阐明,这些样品是在高铁酸盐(VI),砷酸盐和磷酸盐的不同重量比下制备的。这些结果显示了磷酸盐对砷去除机理的三个关键影响。在低磷:由于重量比(高达1:1),砷酸根离子掺入的晶体结构了γ-Fe 2 ö 3发现/γ-FeOOH纳米颗粒被磷酸盐的存在抑制。因此,砷主要吸附到γ-Fe的表面2 ö 3 /γ-的FeOOH纳米颗粒。P:As重量比的进一步增加(大于1:1)导致砷酸盐和磷酸盐吸附之间的竞争。与磷酸盐离子的浓度增加,砷酸盐的γ-Fe的表面上的数字2 ö 3 /γ-的FeOOH纳米颗粒减少。最后,铁的络合(III)离子与磷酸盐离子的发生,导致在去除砷效率的下降,这导致从沉淀的含量较低了γ-Fe 2 ö 3/γ-FeOOH纳米粒子。在使用高铁酸盐(VI)除去天然天然水中的砷之前,需要考虑所有这些方面。



























 京公网安备 11010802027423号
京公网安备 11010802027423号