当前位置:
X-MOL 学术
›
Asian J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Iron‐Catalyzed Directed C−H Silylation of Pivalophenone N−H Imines
Asian Journal of Organic Chemistry ( IF 2.7 ) Pub Date : 2018-06-01 , DOI: 10.1002/ajoc.201800171 Wengang Xu 1 , Jie Hui Pek 1 , Naohiko Yoshikai 1
Asian Journal of Organic Chemistry ( IF 2.7 ) Pub Date : 2018-06-01 , DOI: 10.1002/ajoc.201800171 Wengang Xu 1 , Jie Hui Pek 1 , Naohiko Yoshikai 1
Affiliation
|
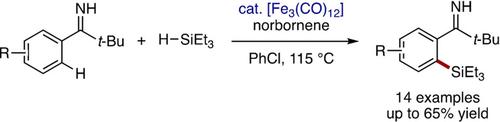
An iron‐catalyzed, N−H imine‐directed arene C−H silylation reaction with hydrosilane has been achieved. Pivalophenone N−H imine undergoes ortho C−H silylation with triethylsilane in the presence of [Fe3(CO)12] as a catalyst and norbornene as a hydrogen acceptor. The product can be readily transformed into ortho‐silylated benzonitrile via conversion of the imine functionality to a cyano group under peroxide photolysis.
中文翻译:

铁催化新戊苯乙胺亚胺的CH H硅烷化反应
已实现了与氢硅烷的铁催化,NH亚胺定向的芳烃CH甲硅烷基化反应。在[Fe 3(CO)12 ]作为催化剂和降冰片烯作为氢受体的存在下,新戊苯甲酮NH亚胺与三乙基硅烷进行邻位CH H甲硅烷基化。通过在过氧化物光解作用下将亚胺官能团转化为氰基,可以很容易地将产物转化为邻甲硅烷基化的苄腈。
更新日期:2018-06-01
中文翻译:

铁催化新戊苯乙胺亚胺的CH H硅烷化反应
已实现了与氢硅烷的铁催化,NH亚胺定向的芳烃CH甲硅烷基化反应。在[Fe 3(CO)12 ]作为催化剂和降冰片烯作为氢受体的存在下,新戊苯甲酮NH亚胺与三乙基硅烷进行邻位CH H甲硅烷基化。通过在过氧化物光解作用下将亚胺官能团转化为氰基,可以很容易地将产物转化为邻甲硅烷基化的苄腈。



























 京公网安备 11010802027423号
京公网安备 11010802027423号