Bioorganic Chemistry ( IF 5.1 ) Pub Date : 2018-05-04 , DOI: 10.1016/j.bioorg.2018.05.002 Ratchanok Pingaew , Veda Prachayasittikul , Nuttapat Anuwongcharoen , Supaluk Prachayasittikul , Somsak Ruchirawat , Virapong Prachayasittikul
|
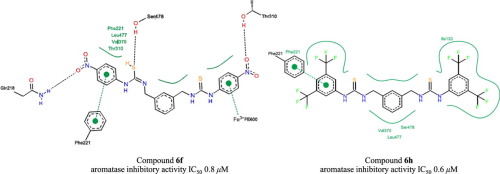
A three series of thioureas, monothiourea type I (4a–g), 1,4-bisthiourea type II (5a–h) and 1,3-bisthiourea type III (6a–h) were synthesized. Their aromatase inhibitory activities have been evaluated. Interestingly, eight thiourea derivatives (4e, 5f–h, 6d, 6f–h) exhibited the aromatase inhibitory activities with IC50 range of 0.6–10.2 μM. The meta-bisthiourea bearing 4-NO2 group (6f) and 3,5-diCF3 groups (6h) were shown to be the most potent compounds with sub-micromolar IC50 values of 0.8 and 0.6 μM, respectively. Molecular docking also revealed that one of the thiourea moieties of these two compounds could mimic steroidal backbone of the natural androstenedione (ASD) via hydrophobic interactions with enzyme residues (Val370, Leu477, Thr310, and Phe221 for 6f, Val370, Leu477, Ser478, and Ile133 for 6h). This is the first time that the bisthioureas have been reported for their potential to be developed as aromatase inhibitors, in which the 4-NO2 and 3,5-diCF3 analogs have been highlighted as promising candidates.
中文翻译:

N,N'-二取代硫脲衍生物作为新型芳香酶抑制剂的合成与分子对接
合成了三个系列的硫脲:I型单硫脲(4a – g),II型1,4-双硫脲(5a – h)和III型1,3-双硫脲(6a – h)。已经评估了它们的芳香酶抑制活性。有趣的是,八种硫脲衍生物(4e,5f–h,6d,6f–h)表现出芳香化酶抑制活性,IC 50范围为0.6–10.2μM。所述元-bisthiourea轴承4-NO 2基(6F)和3,5- DICF 3组(6H)被证明是最有效的化合物,亚微摩尔IC 50值分别为0.8和0.6μM。分子对接还揭示了这两种化合物的硫脲部分之一可以通过与6f的Val370,Leu477,Thr310和Phe221酶残基,Val370,Leu477,Ser478和Ile133 6h)。这是首次报道双硫脲被开发为芳香酶抑制剂的潜力,其中4-NO 2和3,5-diCF 3类似物已被强调为有前途的候选物。


























 京公网安备 11010802027423号
京公网安备 11010802027423号