European Journal of Medicinal Chemistry ( IF 6.7 ) Pub Date : 2018-05-12 , DOI: 10.1016/j.ejmech.2018.04.062 Francesca Curreli , Dmitry S. Belov , Young Do Kwon , Ranjith Ramesh , Anna M. Furimsky , Kathleen O'Loughlin , Patricia C. Byrge , Lalitha V. Iyer , Jon C. Mirsalis , Alexander V. Kurkin , Andrea Altieri , Asim K. Debnath
|
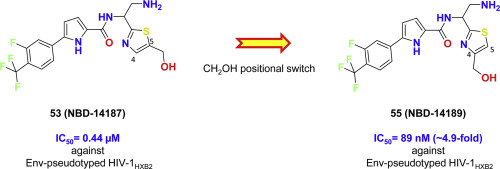
We are continuing our concerted effort to optimize our first lead entry antagonist, NBD-11021, which targets the Phe43 cavity of the HIV-1 envelope glycoprotein gp120, to improve antiviral potency and ADMET properties. In this report, we present a structure-based approach that helped us to generate working hypotheses to modify further a recently reported advanced lead entry antagonist, NBD-14107, which showed significant improvement in antiviral potency when tested in a single-cycle assay against a large panel of Env-pseudotyped viruses. We report here the synthesis of twenty-nine new compounds and evaluation of their antiviral activity in a single-cycle and multi-cycle assay to derive a comprehensive structure-activity relationship (SAR). We have selected three inhibitors with the high selectivity index for testing against a large panel of 55 Env-pseudotyped viruses representing a diverse set of clinical isolates of different subtypes. The antiviral activity of one of these potent inhibitors, 55 (NBD-14189), against some clinical isolates was as low as 63 nM. We determined the sensitivity of CD4-binding site mutated-pseudoviruses to these inhibitors to confirm that they target HIV-1 gp120. Furthermore, we assessed their ADMET properties and compared them to the clinical candidate attachment inhibitor, BMS-626529. The ADMET data indicate that some of these new inhibitors have comparable ADMET properties to BMS-626529 and can be optimized further to potential clinical candidates.
中文翻译:

基于结构的铅优化可提高针对HIV-1 gp120的苯基-1H-吡咯-羧酰胺进入抑制剂的抗病毒效力和ADMET特性
我们正在继续共同努力,以优化我们的第一个铅进入拮抗剂NBD-11021,其靶向HIV-1包膜糖蛋白gp120的Phe43腔,以提高抗病毒效力和ADMET特性。在本报告中,我们提出了一种基于结构的方法,该方法帮助我们产生了可行的假设,以进一步修改最近报道的先进的铅进入拮抗剂NBD-14107,该抑制剂在单周期试验中针对抗大型的Env假型病毒。我们在这里报告了29种新化合物的合成及其在单周期和多周期分析中的抗病毒活性评估,以得出全面的构效关系(SAR)。我们选择了三种具有高选择性指数的抑制剂,以针对代表一组不同亚型临床分离株的55种Env假型病毒进行测试。这些有效抑制剂之一的抗病毒活性,55(NBD-14189)对某些临床分离株的抗药性低至63 nM。我们确定了CD4结合位点突变型假病毒对这些抑制剂的敏感性,以确认它们靶向HIV-1 gp120。此外,我们评估了它们的ADMET特性,并将其与临床候选附着抑制剂BMS-626529进行了比较。ADMET数据表明,这些新型抑制剂中的某些具有与BMS-626529相当的ADMET特性,可以针对潜在的临床候选者进行进一步优化。



























 京公网安备 11010802027423号
京公网安备 11010802027423号