Electrochemistry Communications ( IF 5.4 ) Pub Date : 2018-05-11 , DOI: 10.1016/j.elecom.2018.05.014 Jiakang Qu , Hongwei Xie , Qiushi Song , Zhiqiang Ning , Haijia Zhao , Huayi Yin
|
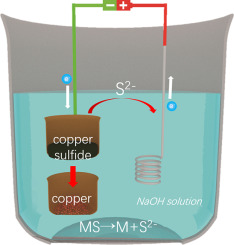
Extraction of metals from their sulfide minerals without emitting SO2 could realize an environment-friendly metallurgical route. Here we demonstrate an electrochemical pathway to reduce solid copper sulfide to copper powder in a 50 wt.% NaOH solution. Unlike oxides, most sulfides are semiconductors or electrical conductors and have less formation energy than that of oxides, resulting in a small polarization during electrolysis and less theoretical dissociation potentials. The use of the strongly alkaline solution suppresses the generation of H2, and the electrolyte could dissolve S2− and then shuttle the S2− between cathode and anode. Moreover, a transparent cell was assembled to directly observe the reduction process of solid copper sulfide. On the anode side, the irreversibility of anodic reactions prevents the parasitic reactions, thereby ensuring a current efficiency over 90%. This electrochemical pathway could be employed for extracting various metals from their sulfides without SO2 emissions.
中文翻译:

固体硫化铜在强碱性溶液中的电化学脱硫
从其硫化物矿物中提取金属而不释放SO 2可以实现环境友好的冶金路线。在这里,我们展示了一种电化学途径,可以将固体硫化铜在50%(重量)的NaOH溶液中还原成铜粉。与氧化物不同,大多数硫化物是半导体或电导体,比氧化物具有更少的形成能,从而导致电解过程中的极化较小,理论上的解离电位也较小。使用强碱性溶液可以抑制H 2的产生,并且电解质可以溶解S 2−,然后使S 2−穿梭。在阴极和阳极之间。此外,组装透明电池以直接观察固体硫化铜的还原过程。在阳极方面,阳极反应的不可逆性阻止了寄生反应,从而确保了超过90%的电流效率。该电化学途径可用于从其硫化物中提取各种金属而无SO 2排放。


























 京公网安备 11010802027423号
京公网安备 11010802027423号