Chemical Physics Letters ( IF 2.8 ) Pub Date : 2018-05-10 , DOI: 10.1016/j.cplett.2018.05.019 Sara Hany , Mira Skaf , Samer Aouad , Cédric Gennequin , Madona Labaki , Edmond Abi-Aad , Antoine Aboukaïs
|
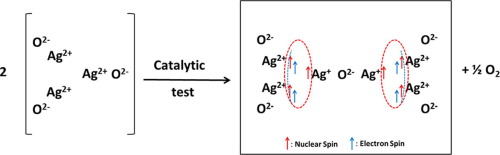
Ag2+(b) species were detected by electron paramagnetic resonance (EPR) on silver loaded over ceria by impregnation in a previous work. Contrasting the EPR simulation with the experimental measurements evidenced that, upon propene oxidation, the Ag2+(b) species transform into two clusters each containing 2 Ag2+ and 1 Ag+ species. This cluster is composed of two parallel electron spins (dimer) and three nuclear spins (trimer). Such a result led us to suggest that initially, Ag2+(b) species clusters were formed by three parallel electron spins. The ferromagnetic character of Ag2+(b) species may explain their catalytic performance towards total oxidation of propylene.
中文翻译:

EPR模拟,以证实在丙烯氧化反应后10%Ag / CeO 2催化剂表面上会形成Ag 6 O 5络合物
在先前的工作中,通过浸渍在氧化铈上的银上,通过电子顺磁共振(EPR)检测到Ag 2+ (b)物种。将EPR模拟与实验测量结果进行对比,结果表明,丙烯氧化后,Ag 2+ (b)物种转变为两个簇,每个簇包含2个Ag 2+和1个Ag +物种。该簇由两个平行的电子自旋(二聚体)和三个核自旋(三聚体)组成。这样的结果使我们认为,最初,Ag 2+ (b)物种簇是由三个平行的电子自旋形成的。Ag 2+ (b)的铁磁特性 物种可能解释了它们对丙烯完全氧化的催化性能。


























 京公网安备 11010802027423号
京公网安备 11010802027423号