当前位置:
X-MOL 学术
›
Sustain. Energy Fuels
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Displacement reduction routed Au–Pt bimetallic nanoparticles: a highly durable electrocatalyst for methanol oxidation and oxygen reduction†
Sustainable Energy & Fuels ( IF 5.6 ) Pub Date : 2018-05-10 00:00:00 , DOI: 10.1039/c7se00565b N. S. K. Gowthaman 1, 2, 3, 4, 5 , Bharathi Sinduja 1, 2, 3, 4, 5 , Sekar Shankar 1, 2, 3, 4, 5 , S. Abraham John 1, 2, 3, 4, 5
Sustainable Energy & Fuels ( IF 5.6 ) Pub Date : 2018-05-10 00:00:00 , DOI: 10.1039/c7se00565b N. S. K. Gowthaman 1, 2, 3, 4, 5 , Bharathi Sinduja 1, 2, 3, 4, 5 , Sekar Shankar 1, 2, 3, 4, 5 , S. Abraham John 1, 2, 3, 4, 5
Affiliation
|
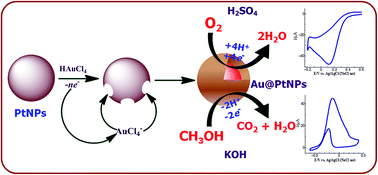
In this paper, a new galvanic displacement reduction (GDR) approach was demonstrated for Au–PtNPs synthesis with different Pt : Au compositions in an aqueous medium. PtNPs were initially synthesized by the reduction of H2PtCl6 using trisodium citrate and sodium borohydride. Addition of various concentrations of HAuCl4 to PtNPs leads to the formation of Au–PtNPs, which follows the GDR between Pt(0) and AuCl4− ions. The formation of Au–PtNPs was monitored by UV-vis spectroscopy by tuning the mole ratio of Pt : Au. HR-TEM images showed that the Au–PtNPs were spherical with 11 nm diameter. HR-TEM, XRD and XPS analysis showed that the formed Au–PtNPs were in the form of a core–shell structure. The colloidal Au–PtNPs were then attached on a glassy carbon (GC) electrode via a 1,6-hexanediamine linker for the methanol oxidation reaction (MOR) and oxygen reduction reaction (ORR). The attachment of Au–PtNPs was further confirmed by XRD, line scanning coupled with energy-dispersive X-ray spectroscopy (EDS) and cyclic voltammetry (CV). The Au–PtNPs modified electrode exhibits a higher heterogeneous electron transfer rate constant of 4.12 × 10−3 cm s−1 than bare (1.01 × 10−4 cm s−1) and PtNP (1.77 × 10−4 cm s−1) modified GC electrodes. Further, the Au–PtNPs modified electrode exhibited a composition dependent activity towards the MOR and ORR. It was found that the modified electrode with a Pt : Au ratio of 1 : 0.09 shows 8 times more sensitive oxidation for the MOR when compared to a commercial Pt/C catalyst. The present Au–PtNPs catalyst exhibits a greatly enhanced catalytic activity in terms of mass activity (132 mA mg Pt−1) and excellent stability relative to the commercial Pt/C catalyst.
中文翻译:

减少位移的Au-Pt双金属纳米颗粒:一种用于甲醇氧化和氧还原的高度耐用的电催化剂†
在本文中,展示了一种在水介质中具有不同Pt:Au组成的Au-PtNPs合成的新的电流位移减少(GDR)方法。最初通过使用柠檬酸三钠和硼氢化钠还原H 2 PtCl 6来合成PtNP 。在PtNPs中添加各种浓度的HAuCl 4会导致Au–PtNPs的形成,其遵循Pt(0)和AuCl 4 −之间的GDR离子。通过调节Pt:Au的摩尔比,通过紫外-可见光谱法监测Au-PtNPs的形成。HR-TEM图像显示Au-PtNPs是球形的,直径为11 nm。HR-TEM,XRD和XPS分析表明,形成的Au-PtNPs为核-壳结构形式。然后将胶体Au–PtNPs通过1,6-己二胺连接剂附着在玻璃碳(GC)电极上,以进行甲醇氧化反应(MOR)和氧还原反应(ORR)。XRD,线扫描,能量色散X射线光谱法(EDS)和循环伏安法(CV)进一步证实了Au-PtNPs的附着。Au-PtNPs修饰电极比裸露电极(1.01×10)表现出更高的异质电子传递速率常数,为4.12×10 -3 cm s -1-4 cm s -1)和PtNP(1.77×10 -4 cm s -1)修饰的GC电极。此外,Au-PtNPs修饰电极对MOR和ORR表现出依赖成分的活性。已经发现,与市售的Pt / C催化剂相比,Pt∶Au比率为1∶0.09的改性电极对MOR显示出8倍的MOR敏感氧化。相对于商业化的Pt / C催化剂,目前的Au-PtNPs催化剂在质量活性(132 mA mg Pt -1)方面显示出大大增强的催化活性。
更新日期:2018-05-10
中文翻译:

减少位移的Au-Pt双金属纳米颗粒:一种用于甲醇氧化和氧还原的高度耐用的电催化剂†
在本文中,展示了一种在水介质中具有不同Pt:Au组成的Au-PtNPs合成的新的电流位移减少(GDR)方法。最初通过使用柠檬酸三钠和硼氢化钠还原H 2 PtCl 6来合成PtNP 。在PtNPs中添加各种浓度的HAuCl 4会导致Au–PtNPs的形成,其遵循Pt(0)和AuCl 4 −之间的GDR离子。通过调节Pt:Au的摩尔比,通过紫外-可见光谱法监测Au-PtNPs的形成。HR-TEM图像显示Au-PtNPs是球形的,直径为11 nm。HR-TEM,XRD和XPS分析表明,形成的Au-PtNPs为核-壳结构形式。然后将胶体Au–PtNPs通过1,6-己二胺连接剂附着在玻璃碳(GC)电极上,以进行甲醇氧化反应(MOR)和氧还原反应(ORR)。XRD,线扫描,能量色散X射线光谱法(EDS)和循环伏安法(CV)进一步证实了Au-PtNPs的附着。Au-PtNPs修饰电极比裸露电极(1.01×10)表现出更高的异质电子传递速率常数,为4.12×10 -3 cm s -1-4 cm s -1)和PtNP(1.77×10 -4 cm s -1)修饰的GC电极。此外,Au-PtNPs修饰电极对MOR和ORR表现出依赖成分的活性。已经发现,与市售的Pt / C催化剂相比,Pt∶Au比率为1∶0.09的改性电极对MOR显示出8倍的MOR敏感氧化。相对于商业化的Pt / C催化剂,目前的Au-PtNPs催化剂在质量活性(132 mA mg Pt -1)方面显示出大大增强的催化活性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号