当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Evolution of the Synthesis of AMPK Activators for the Treatment of Diabetic Nephropathy: From Three Preclinical Candidates to the Investigational New Drug PF-06409577
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2018-05-10 00:00:00 , DOI: 10.1021/acs.oprd.8b00059 Aaron C. Smith 1 , Daniel W. Kung 1 , Andre Shavnya 1 , Thomas A. Brandt 1 , Philip D. Dent 1 , Nathan E. Genung 1 , Shawn Cabral 1 , Jane Panteleev 1 , Michael Herr 1 , Ka Ning Yip 2 , Gary E. Aspnes 2 , Edward L. Conn 1 , Matthew S. Dowling 1 , David J. Edmonds 2 , Ian D. Edmonds 3 , Dilinie P. Fernando 1 , Paul M. Herrinton 4 , Nandell F. Keene 1 , Sophie Y. Lavergne 1 , Qifang Li 1 , Jana Polivkova 1 , Colin R. Rose 1 , Benjamin A. Thuma 1 , Michael G. Vetelino 1 , Guoqiang Wang 1 , John D. Weaver 1 , Daniel W. Widlicka 1 , Kristin E. Price Wiglesworth 1 , Jun Xiao 1 , Todd Zahn 4 , Yingxin Zhang 1
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2018-05-10 00:00:00 , DOI: 10.1021/acs.oprd.8b00059 Aaron C. Smith 1 , Daniel W. Kung 1 , Andre Shavnya 1 , Thomas A. Brandt 1 , Philip D. Dent 1 , Nathan E. Genung 1 , Shawn Cabral 1 , Jane Panteleev 1 , Michael Herr 1 , Ka Ning Yip 2 , Gary E. Aspnes 2 , Edward L. Conn 1 , Matthew S. Dowling 1 , David J. Edmonds 2 , Ian D. Edmonds 3 , Dilinie P. Fernando 1 , Paul M. Herrinton 4 , Nandell F. Keene 1 , Sophie Y. Lavergne 1 , Qifang Li 1 , Jana Polivkova 1 , Colin R. Rose 1 , Benjamin A. Thuma 1 , Michael G. Vetelino 1 , Guoqiang Wang 1 , John D. Weaver 1 , Daniel W. Widlicka 1 , Kristin E. Price Wiglesworth 1 , Jun Xiao 1 , Todd Zahn 4 , Yingxin Zhang 1
Affiliation
|
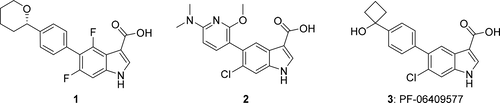
Indole acids 1, 2, and 3 are potent 5′-adenosine monophosphate-activated protein kinase (AMPK) activators for the potential treatment of diabetic nephropathy. Compounds 1–3 were scaled to supply material for preclinical studies, and indole 3 was selected for advancement to first-in-human clinical trials and scaled to kilogram quantities. The progression of the synthesis strategy for these AMPK activators is described, as routes were selected for efficient structure–activity relationship generation and then improved for larger scales. The developed sequences employed practical isolations of intermediates and APIs, reproducible cross-coupling, hydrolysis, and other transformations, and enhanced safety and purity profiles and led to the production of 40–50 g of 1 and 2 and 2.4 kg of 3. Multiple polymorphs of 3 were observed, and conditions for the reproducible formation of crystalline material suitable for clinical development were identified.
中文翻译:

用于治疗糖尿病性肾病的AMPK激活剂合成的演变:从三个临床前候选药物到正在研究的新药PF-06409577
吲哚羧酸1,2,和3是有效的5'-腺苷对于糖尿病性肾病的潜在治疗单磷酸活化蛋白激酶(AMPK)激活剂。化合物1 - 3分别缩放以用于临床前研究提供材料,和吲哚3被选择用于人类首创的临床试验,并按千克量定标。描述了这些AMPK激活剂的合成策略的进展,为有效的结构-活性关系生成选择了路线,然后针对更大的规模对其进行了改进。所开发的序列采用了中间体和API的实际分离方法,可重现的交叉偶联,水解和其他转化方法,并提高了安全性和纯度,从而生产了40–50 g的1和2和2.4 kg的3。观察到3的多个多晶型物,并确定了可重复形成适合临床发展的结晶物质的条件。
更新日期:2018-05-10
中文翻译:

用于治疗糖尿病性肾病的AMPK激活剂合成的演变:从三个临床前候选药物到正在研究的新药PF-06409577
吲哚羧酸1,2,和3是有效的5'-腺苷对于糖尿病性肾病的潜在治疗单磷酸活化蛋白激酶(AMPK)激活剂。化合物1 - 3分别缩放以用于临床前研究提供材料,和吲哚3被选择用于人类首创的临床试验,并按千克量定标。描述了这些AMPK激活剂的合成策略的进展,为有效的结构-活性关系生成选择了路线,然后针对更大的规模对其进行了改进。所开发的序列采用了中间体和API的实际分离方法,可重现的交叉偶联,水解和其他转化方法,并提高了安全性和纯度,从而生产了40–50 g的1和2和2.4 kg的3。观察到3的多个多晶型物,并确定了可重复形成适合临床发展的结晶物质的条件。


























 京公网安备 11010802027423号
京公网安备 11010802027423号