Journal of Fluorine Chemistry ( IF 1.9 ) Pub Date : 2018-05-08 , DOI: 10.1016/j.jfluchem.2018.05.003 Sumanta Mukherjee , Smruti Dash
|
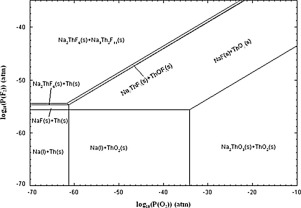
The standard molar Gibbs energy of formation of Na2ThF6(s) has been determined using an e.m.f. technique. The fluoride cell: (-) Pt, NiO(s) + NiF2(s)│CaF2(s)│ThOF2(s)+ Na2ThF6(s) + NaF(s), Pt (+) has been constructed to measure Gibbs energy of formation of Na2ThF6(s) using CaF2(s) as solid electrolyte. From the measured e.m.f. values of the cell and required Gibbs energy values from the literature, ΔrG°m (Na2ThF6, s, T) has been calculated as: ΔfG°m (Na2ThF6, s, T) (kJ mol−1) ± 10 = − 3378 + 0.5296·(T/K) (700 K ≤ T ≤ 945 K). The heat capacity of Na2ThF6(s) was measured with Differential Scanning Calorimeter in the temperature range 300–800 K. The enthalpy of formation of Na2ThF6(s) at 298.15 K has been calculated by the second method from the measured Gibbs energy and heat capacity data. The chemical potential diagram of Na-Th-F-O system has been computed based on measured data in this study and required data from the literature. Based on the thermodynamic data, melting points and compositions of several potential salt mixtures have been suggested for Molten Salt Reactors.
中文翻译:

NaF-ThF 4系统和熔融盐反应堆燃料盐的热力学研究
已使用电动势技术确定了Na 2 ThF 6(s)的标准摩尔吉布斯形成能。氟化物细胞:( - )的Pt,氧化镍(多个)+ NIF 2(S)│CaF 2(S)│ThOF 2(S)+的Na 2 THF 6(S)+氟化钠(S),铂(+)具有用CaF 2(s)作为固体电解质来测量Na 2 ThF 6(s)的吉布斯形成能。根据电池的测得的电动势值和文献中所需的吉布斯能量值,已将Δr G° m(Na 2 ThF 6,s,T)计算为:Δf G° m(Na 2 ThF 6,s,T)(kJ mol -1)±10 =-3378 + 0.5296·(T / K)(700 K≤T≤945 K)。的Na的热容量2 THF 6(S),用差示扫描量热计测得的温度范围300-800 K.形成的Na焓2 THF 6通过第二种方法,从实测的吉布斯能量和热容量数据计算出298.15 K时的(s)。Na-Th-FO系统的化学势图已基于本研究中的测量数据和文献中所需的数据进行了计算。根据热力学数据,已提出熔融盐反应器的几种潜在盐混合物的熔点和组成。


























 京公网安备 11010802027423号
京公网安备 11010802027423号